
Our Team

Background
Scoliosis is an abnormal lateral curvature and rotation of the spine. There is a 2-3% prevalence worldwide. In Ghana, 138000 to 207000 adolescents may be affected by using this statistic.
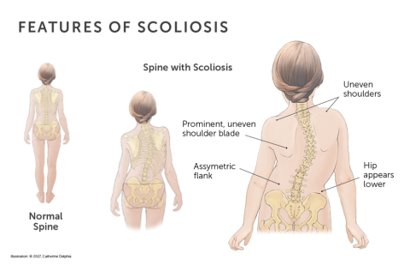
Problem
In Ghana 138000 to 207000 adolescents may be affected. More severe cases seen in low resource settings due to: (1) lack of health care, (2) lack of awareness/education, (3) lack of screening programs, and (4) lack of affordable technology. This leads to undiagnosed scoliosis and disease progression which causes: (1) cardiopulmonary issues and pain, (2) increased cost of treatment, and (3) poor quality of life.
Biodesign Statement
A way to screen for the presence of scoliosis in adolescents to aid in determining an optimal treatment plan to minimize future complications and cost of care.
Current Screening Methods
Adam’s forward bending test (AFBT): Patient bends forward at the waist while the examiner observes the spine from behind. Widely used but has a high false-negative rate. Not a definitive diagnostic tool, and evaluation by a trained medical professional is necessary.
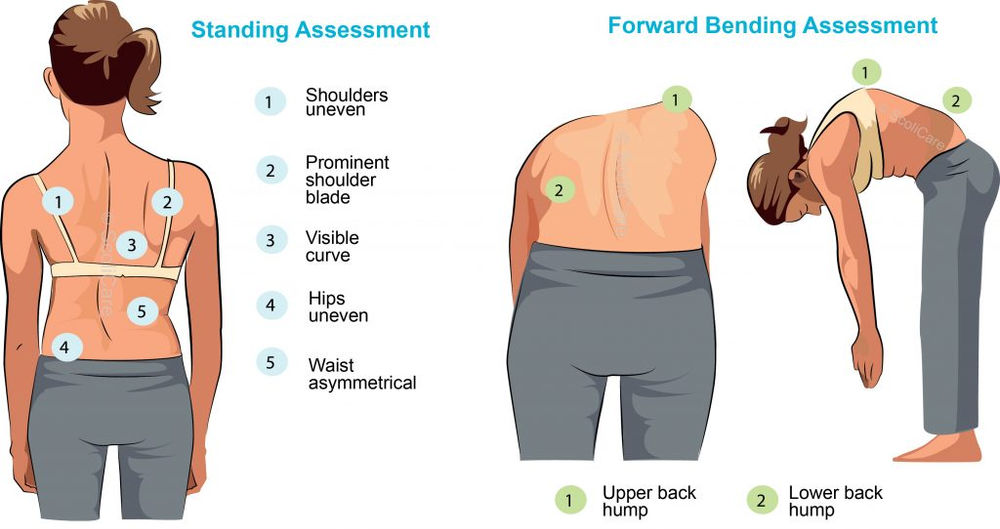
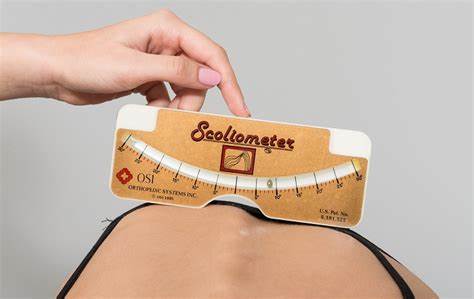
Scoliometer: handheld device used to measure the angle of trunk rotation (ATR). It is placed on the patient’s back, and examiner reads the measurement. Often used in conjunction with other diagnostic tools, such as X-rays, to monitor and make treatment plans. Accuracy of measurement is affected by examiner’s technique and the patient’s body position, and so should be used by a trained medical professional.
Our Solution
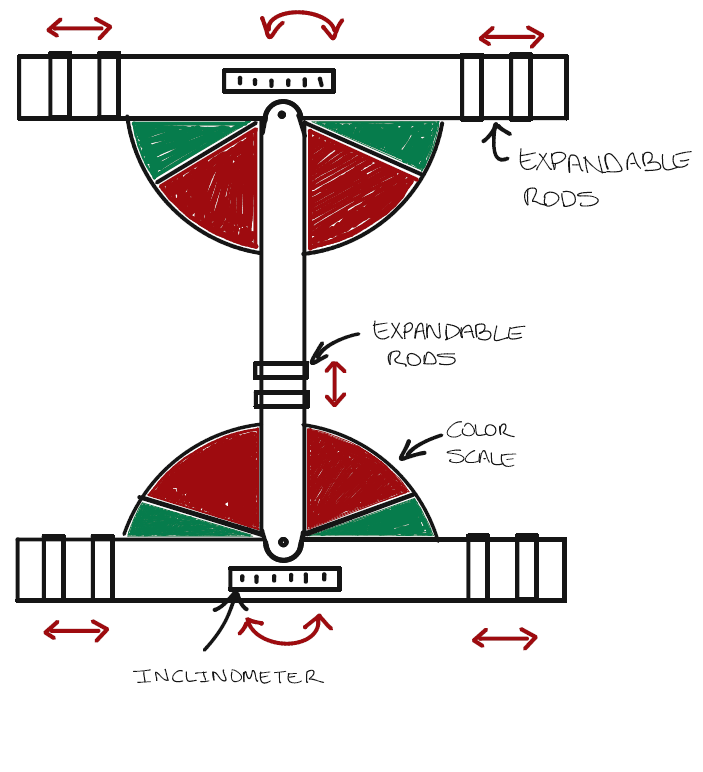
Our solution is a fully-mechanical and low cost-device that can be used by anyone regardless of literacy level that indicates abnormal trunk curvature by measuring pelvic tilt and shoulder asymmetry.
Testing
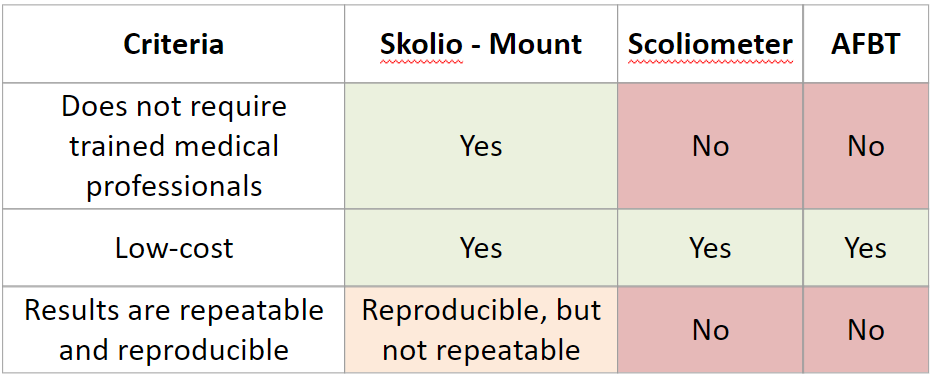
We conducted a Gage R&R analysis to determine whether the results from our device are repeatable by the same screener, and reproducible across screeners. We found that it was reproducible but not repeatable. To increase the repeatability, we would need to add markers on the 4 landmarks of the patient prior to putting the device on them.
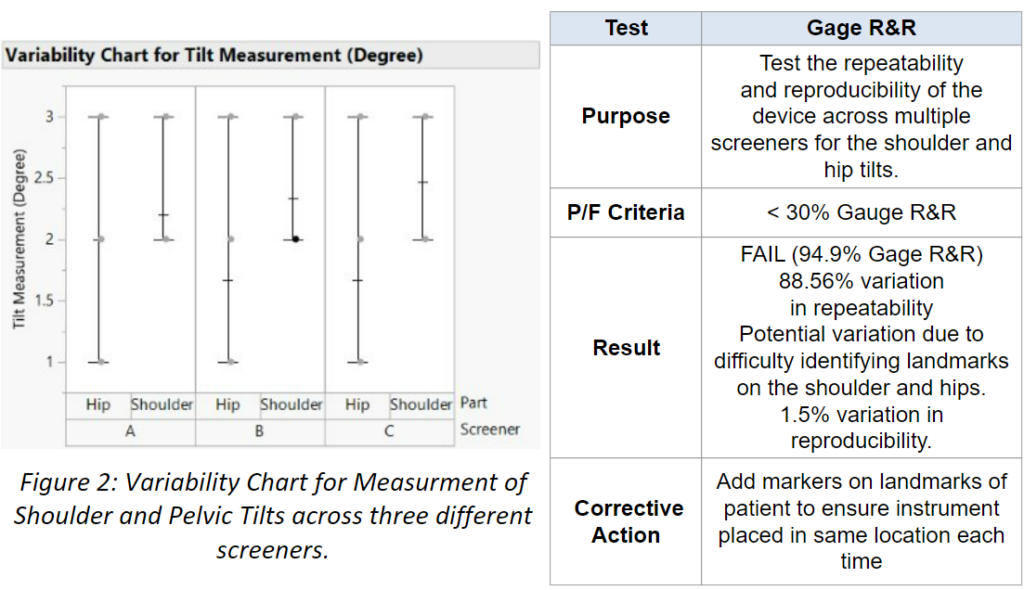
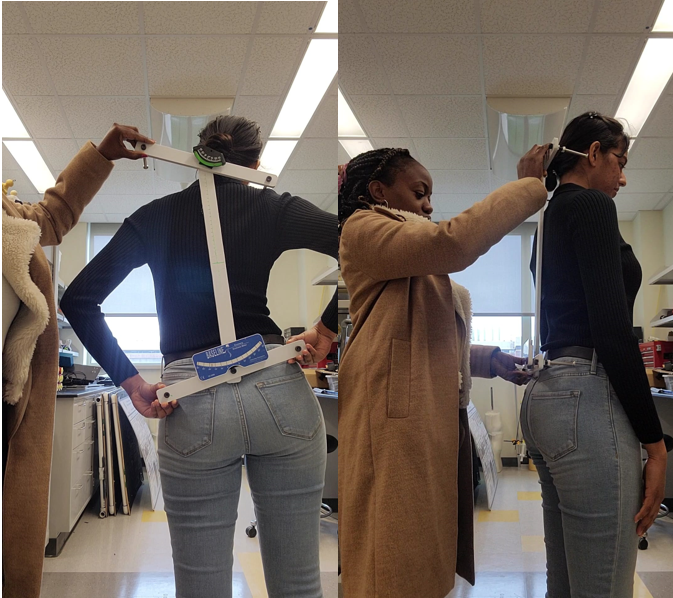
How to use Skolio Mount
1. Ask patient to stand with feet slightly apart.
2. Position the top two extrudes on the highest peak of each of the shoulders.
3. Position the bottom two extrudes on each side of the hips of the patient.
4. Ensure the middle rod is vertical.
5. Determine the reading on the top and bottom inclinometers and determine whether the rods are in the green region (no action necessary) or red region (suggest visit to orthopedics)
Certification and Education
Our system includes a certification and education program along with the device itself to offer communities in low resource areas an opportunity to raise awareness on scoliosis, learn about Skolio Mount, and implement a screening program as they see fit for their needs.
Certification
- To ensure Skolio Mount is being used correctly and appropriate guidance is given.
- Consists of 1-day in person training and educational modules to learn about scoliosis and the screening process.
Education Program
- Meant for the greater community to educate and raise awareness on scoliosis.
- Acts as an aid to build trust by explaining how Skolio-Mount works.
Future Improvements
- Place markers on patient to align with extrudes
- Add radiation-free imaging to automate calculations
- Conduct larger study with improved prototype to determine Gage R&R
- Make entire system available in multiple languages
Impact Size and Regulatory Considerations
Impact Size
- 138-207k Ghanian adolescents may be affected.
- Most severe cases of scoliosis found in low resource settings.
- No screening programs currently exist.
- Skolio-mount is niche product (eliminate need for surgery ~$13000 cost, by implementing Skolio-Mount ~$250 cost.
Regulatory Considerations
- Class 1 Device
- Would need to adhere to local regulations (in Ghana and elsewhere)
Acknowledgements
Dr. Greg Gdowski – Executive Director of CMTI Program
Martin Gira – University of Rochester Senior Engineer
Dr. Amy Lerner – Academic Director of CMTI Program
Dr. Jonathan J. Stone, M.D – URMC Attending Neurosurgeon
Dr. Nelson, MD – URMC Pediatric Surgeon
Dr. Joan Adamo – Director of Regulatory Support Services
John Boger, J.D. – Patent Lawyer, IP
References
- Negrini, S., Donzelli, S., Aulisa, A. G., Czaprowski, D., Schreiber, S., de Mauroy, J. C., Diers, H., Grivas, T. B., Knott, P., Kotwicki, T., Lebel, A., Marti, C., Maruyama, T., O’Brien, J., Price, N., Parent, E., Rigo, M., Romano, M., Stikeleather, L., … Zaina, F. (2018). 2016 SOSORT guidelines: Orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis and Spinal Disorders, 13(1). https://doi.org/10.1186/s13013-017-0145-8
- Ghana population (live). Worldometer. (2022). Retrieved December 16, 2022, from https://www.worldometers.info/world-population/ghana-population/
- National Health Insurance Authority – National Health Insurance Authority (nhis.gov.ng)
- Med-Peds, B. (2022, June 22). Scoliosis: How to use a scoliometer. BROWN MED-PEDS RESIDENCY. Retrieved December 16, 2022, from https://brownmedpedsresidency.org/scoliosis/
- Côté, P., Kreitz, B. G., Cassidy, J. D., Dzus, A. K., & Martel, J. (1998). A study of the diagnostic accuracy and reliability of the scoliometer and Adamʼs Forward Bend Test. Spine, 23(7), 796–802. https://doi.org/10.1097/00007632-199804010-00011
