Authors
- Anika Callanan
- Miraz A. Sadi
- Quinn Taylor
Mentors
Mitch Anthamatten and Melodie Lawton ( Department of Chemical Engineering)
Abstract
The crystallization speed of a polymer can be a limiting step in the production of products. The sponsor of the project, Danimer Scientific, posed the problem of slow crystallization, and the goal of increasing the crystallization temperature (Tc) of the biodegradable polymer, poly(3-hydroxypropionic acid) (P3HP). A bio-based nucleator was used to increase the polymer’s Tc. A DSC screen was conducted using several bio-based additives where orotic acid (OA) was the most promising candidate. To study the scaled-up production of the polymer, a temperature-controlled mold was designed to study isothermal crystallization during the process.
Introduction
Background:
The production of non-biodegradable plastic is projected to triple over the next decades, potentially leading to a quadrupling in plastic pollution in oceans (The Guardian, 2016).
P3HP is an emerging biodegradable polymer produced by Danimer Scientific.
The polymer has a long crystallization time, prompting the exploration of bio-based additives to accelerate crystallization and reduce processing time.
The Tc of a polymer is the temperature at which the polymer organizes itself into a crystalline structure.
Nucleators decrease the energy barrier for crystallization, increasing the temperature that crystallization occurs because less energy has to be put in to overcome the barrier [1].
Nucleators increase the number of crystallization sites, impacting crystal size and thus, mechanical properties
Certain biobased additives increase the crystallization speed of similar polymers such as polyhydroxyalkanoate [2] and poly(L-lactide) [3].

Objective:
- To utilize a bio-based nucleator to increase the Tc of P3HP, enhancing process efficiency
- To scale up the process of crystallizing the polymer by creating a tool to control isothermal crystallization for an industrial process
Methodology
Full Process
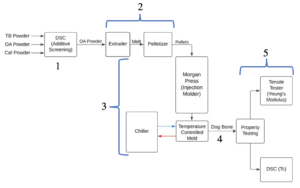
- Additive selection
- Making pellets (done at Danimer Scientific)
- Injection into temperature-controlled mold
- Wait 72 hours after production
- Scaled-up property testing
Characterization of Nucleators
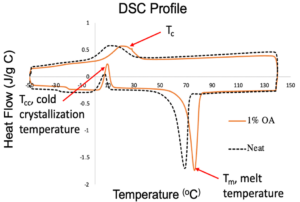
Differential Scanning Calorimetry (DSC) and Polarized Optical Microscope (POM) were used to select an additive and evaluate its effect on P3HP on a bench scale (powder).
- DSC produced an exotherm showing the Tc
- Gel Permeation Chromatography (GPC) evaluated the molar mass degradation after DSC
- POM qualitatively analyzed crystal structures
Scale Up:
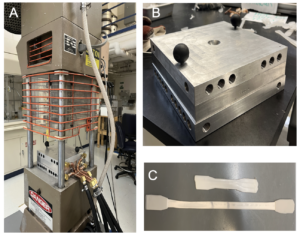
The process was scaled up using a temperature-controlled mold for an injection molder which produced dog bones for mechanical property testing.
- Morgan Press melted pellets and injects into mold
- Cooling channels machined into aluminum block and top of mold
- Tensile testing using Instron to obtain Young’s Modulus (YM)
- DSC run on dog bones show scaled-up Tc changes
Design of Experiments (DOE)
|
Factors |
Levels |
Responses |
|
Mold Temperature |
20˚C, 25˚C, 30˚C (Neat) 35˚C, 40˚C, 45˚C (1% OA) |
Young’s Modulus, Tc |
|
Additive Loading |
0% OA (neat), 1% OA |
Results
Characterization
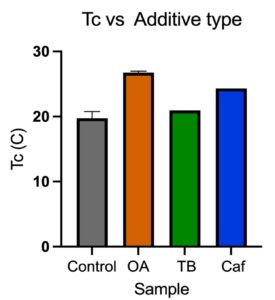
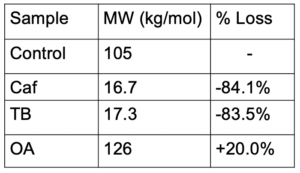
OA was found to be the most effective nucleator for P3HP.
- OA increased Tc more than the other additives
- GPC results showed OA as the only nucleator adding thermal stability
POM images showed OA as an effective nucleator.
- Neat P3HP and 1% OA were heated to 140°C, and cooled to their respective Tc
- OA allowed crystals to form more homogenously than when the polymer lacked a nucleator
- Crystal sizes are smaller with 1% OA, indicating an increased number of nucleation sites, preventing crystals from reaching larger sizes

Scale Up
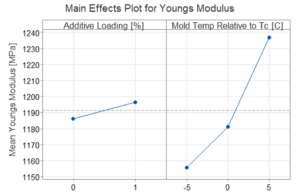
Tensile testing showed:
- Increased mold temperature yielded a higher YM product
- Additive loading had little effect on YM
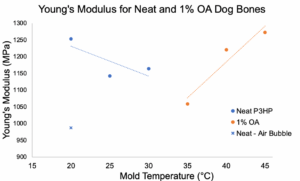
Neat and 1% OA show different YM trends:
- YM for 1% OA increases with increased mold temperature
- Neat remains inconclusive
- Limited material prevented further analysis of neat P3HP
- Air bubble in a neat dog bone resulted in lower YM
- 1% OA showed increasing YM with increasing mold temperature
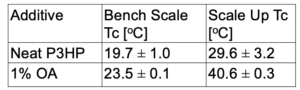 The scaled-up 1% OA polymer product also showed an increase in Tc when compared to the neat dog bones.
The scaled-up 1% OA polymer product also showed an increase in Tc when compared to the neat dog bones.
- Larger increase between neat and OA Tc for scaled-up process compared to bench scale
- Both show statically significant change in Tc
Conclusions and Future Work
Conclusions:
- The Tc of P3HP was increased by 10°C when scaled up
- A 10°C increase in the mold temperature increased the YM of the scaled-up 1% OA product by roughly 20%.
- The development of a scaled-up injection molding process with controlled crystallization using a custom cooling mold
Future Work:
- Compound mixing to evaluate different OA loadings to determine optimal percent to maximize Tc increase
- Optimizing Morgan Press parameters for scale up process (injection pressure, coolant temperature, injection speed) to determine which conditions eliminate air bubbles
- Use a larger sample size to help support the above preliminary results
Acknowledgments
Team Parkour! would like to thank Prof. Lawton and Prof. Anthamatten for advising us this semester. We would like to thank Clair Cunningham, Jeff Lefler, Bill Mildenberger, and Prof. Kelley for assisting in the design and build. Additionally, we want to express our thanks to Kimberly Clem and Ben Carlson for helping us through this project. Finally, we would like to thank Dr. Chris DeRosa and Danimer Scientific for sponsoring this project and the Department of Chemical Engineering for their support.
References
1.Zhang, X., Meng, L., Li, G., Liang, N., Zhang, J., Zhu, Z. and Wang, R. (2016), Effect of nucleating agents on the crystallization behavior and heat resistance of poly(l-lactide). J. Appl. Polym. Sci., 133, 42999.
2.Bledsoe, J., Crane, G., and Locklin, J. Beyond Lattice Matching: The Role of Hydrogen Bonding in Epitaxial Nucleation of Poly(hydroxyalkanoates) by Methylxathines. Applied Polymer Materials. 2023.
3.Qiu, Zhaobin and Li Zhisheng. Effect of Orotic Acid on the Crystallization Kinetics and Morphology of Biodegradable Poly(L-lactide) as an Efficient Nucleating Agent. I&EC Research. 2011.
4.DeRosa, Chris. “Nucleation of Poly(Propiolactone).” Danimer Scientific 2023.
