Women’s Engineering in Life Design, or W.E.L.D, aims to improve outcomes for surgeries commonly undergone by women. We create novel surgical instruments, such as the IllumiCath, that make obstetrics and gynecologic procedures with high risk for complications safer at a lower cost than existing minimally invasive surgical techniques.
Problem
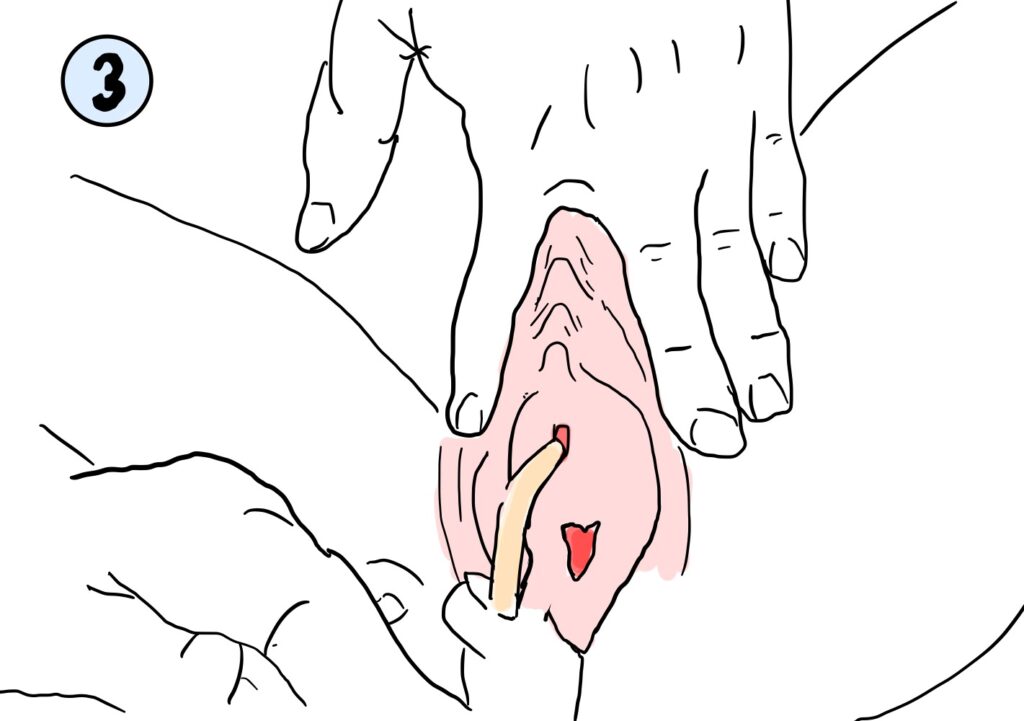
Hysterectomies, or surgical procedures during which part or all the uterus is removed, are the second most common elective procedure done for women in the United States1. While laparoscopic and robotic methods are considered minimally invasive, vaginal hysterectomies are a more minimally invasive procedure in which the uterus is removed through the natural orifice of the vaginal opening only, bringing shorter recovery times, less post-operative time, and no creation of extra incisions in the abdomen. Comorbidities that complicate visualization of the surgical planes (fibroids, extensive endometriosis, etc.), in combination with decreased rates of surgical training in this procedure specifically are factors that can currently increase the risk of harm for this procedure2.
“For my generation of training… people can feel uncomfortable performing a vaginal hysterectomy because the planes between the bladder and peritoneal tissue can be hard to see”
~ Dr. Jared Floch, URMC
Therefore there is a need for an instrument for GYN surgeons that minimizes bladder injury in vaginal hysterectomy patients to avoid urinary complications and bladder repair procedures during the anterior colpotomy.
Problem/Population/Outcome
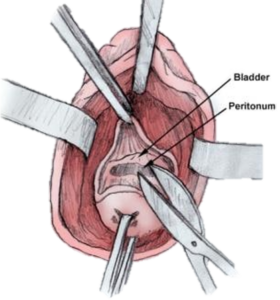
Figure 1: Bladder and Peritoneal Tissue is difficult to differentiate during vaginal hysterectomies
Solution
The IllumiCath highlights bladder tissue and borders to allow gynecologic surgeons to differentiate between neighboring tissue with a faster, more controlled method than traditional injections of methylene blue or indocyanine green dyes.
Our solution utilizes tissue-reveal-technology (TRT) which consists of ABS infused with Indocyanine (ICG) dye that is excited by a laser emitter, and consequently fluoresces. This device is useful with devices such as the Stryker Spy-Phi NIR emitter and camera system, which overlays the fluorescent image with the visible light image to allow providers to differentiate between tissue types.

Figure 2: Final Prototype Iteration for the IllumiCath.

“In brightest day, in blackest night, no evil shall escape my sight. Let those who worship evil’s might. Beware my power – Green Lantern’s light!”
~ Green Lantern, Comic Series3
Main Menu
How to Use the IllumiCath
Prototyping
Design Specifications and Requirements
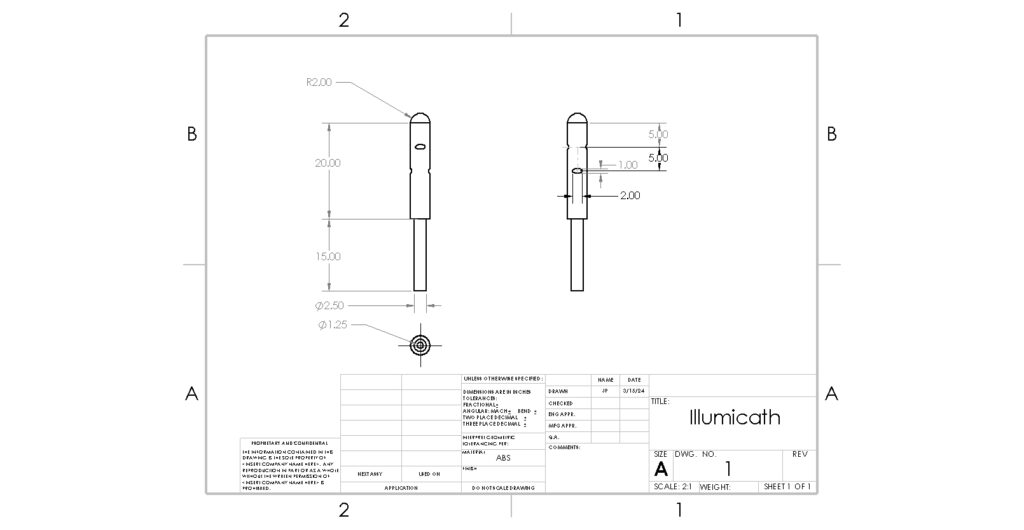
Figure 3: Engineering Drawing of Iteration 1 of the IllumiCath.
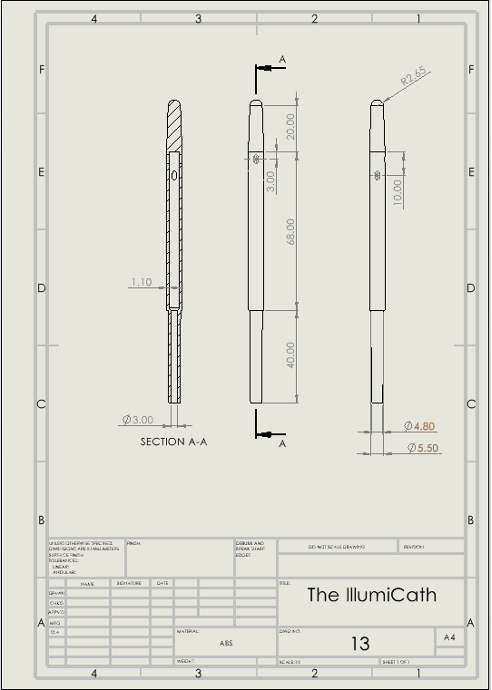
Figure 4: Engineering Drawing of Final Iteration of the IllumiCath.
| Needs | Metrics | Wants | Metrics |
| Device reduces risk of bladder injury | Enhanced ability to avoid damage to bladder than with existing methods/instruments | No extra instruments crowding space | Device replaces or removes instruments currently used in procedure (catheters) |
| Device maintains functionality of existing instruments used | IllumiCath must meet functionality standards of currently used catheters (ASTM F623 – 19) | Comfort for patient during and following procedure | Device is made of materials that do not bring more discomfort than currently used instruments |
Table 1: Needs and wants based on customer discovery, and metrics defined to understand if device fulfills requirements.
3D Modeling and Printing
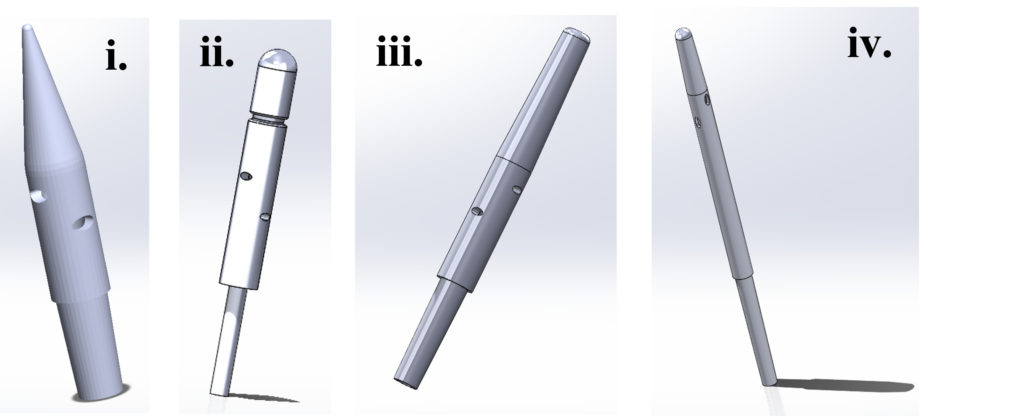
Figure 5: Various Iterations of the IllumiCath. (i) and (ii) were some of the earlier prototypes which evolved to have smoother tips to increase patient comfort and a longer body to increase end user visualization capabilities.
While our preliminary prototyping was completed with 3D printing of TRT, W.E.L.D. would look to utilizing reaction molding or extrusion of silicone-rubber infused with ICG dye for its final mass-manufactured product.
Testing
How will bodily fluids affect the IllumiCath’s performance?

Figure 6: Fluorescence of block of tissue-reveal technology alone.
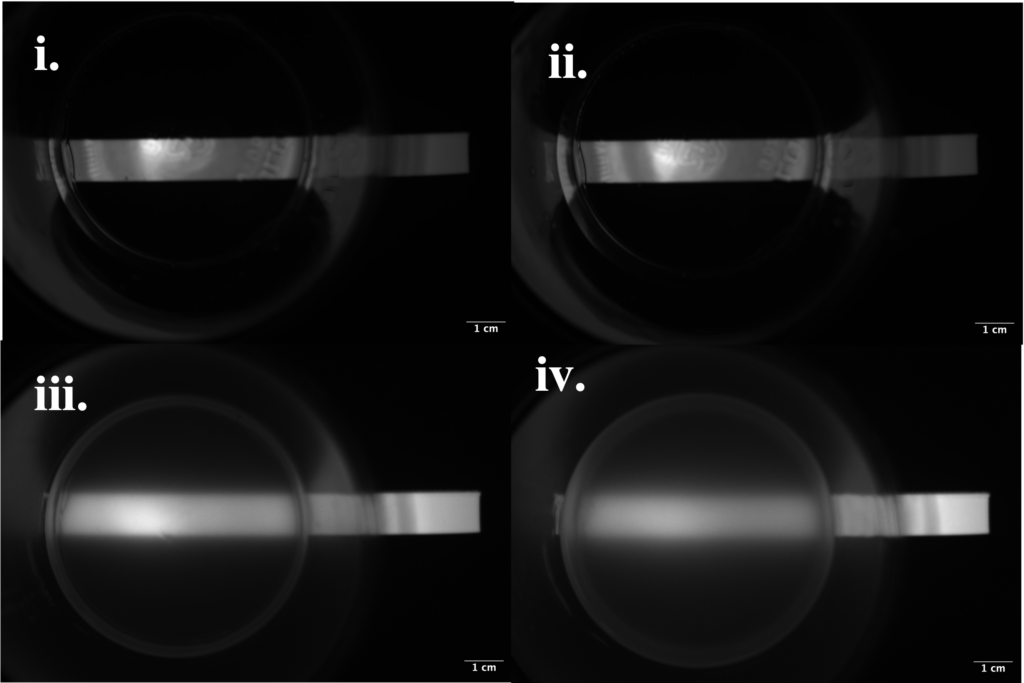
Figure 7: Fluorescence of block of tissue-reveal technology through various opacities.
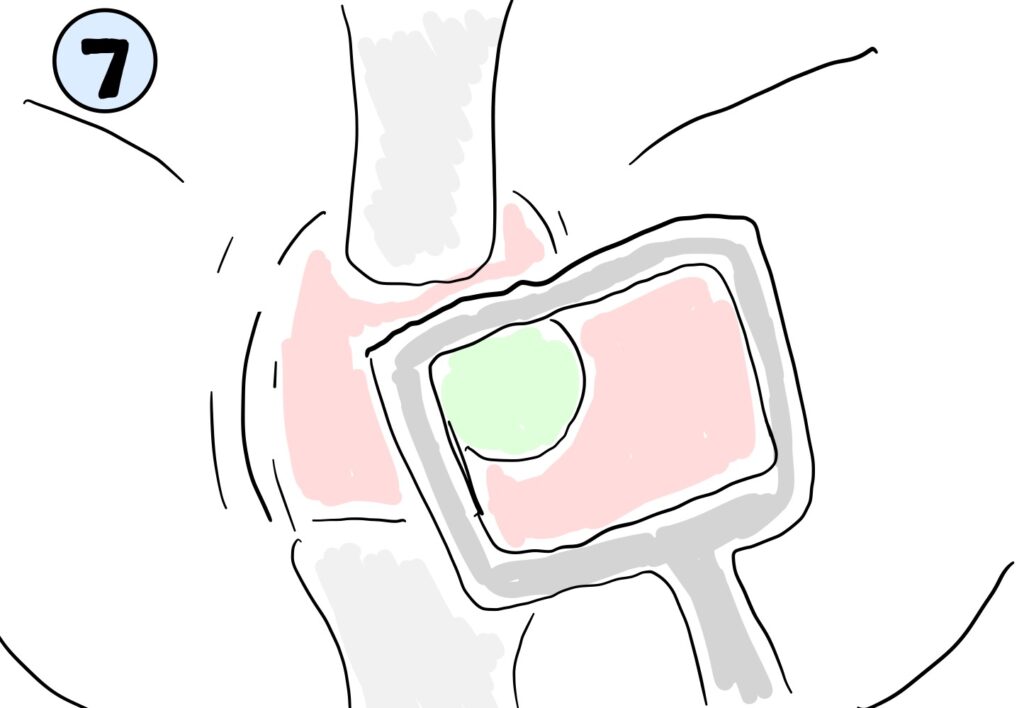
To test the effect different bodily tissues of different moduli and opacities had on the fluorescence of the tissue-reveal-technology, our team conducted testing comparing the relative fluorescence intensities. Fluorescence was compared in water mixed with yellow-food dye to simulate urine at depths of (i) 12 mm and (ii) 17.5 mm, as well as silicone rubber (a more opaque substance) at (iii) 12 mm and (iv) 17.5 mm. The relative visibility of the fluorescence of the material was determined through processing of 16-bit images taken with a near-infrared camera and emitter system in Fiji ImageJ analysis software. A region of interest (ROI) was identified on each image, and from that the average intensity of the ROI was found. This revealed that the simulated urine did not scatter the fluorescence or change its brightness regardless of depth. However, the greater depth of opaque silicone rubber did reduce brightness when compared to a the fluorescence through a smaller amount of opaque material.
Preliminary testing of the IllumiCath in the bladder
To understand if our device possessed enough tissue-reveal-technology in the proper composition to fluoresce through bladder tissue, qualitative analysis of the ability for the IllumiCath to fluoresce through bladder tissue was conducted. However, this length was not long enough to illuminate both the edge of the bladder and the urethra at the same time, which is why another longer iteration was created.
How much light comes through the bladder?
The IVIS system by Caliper Life Sciences was used to quantify the amount of light coming through the cow bladder in units of flux from various prototypes and objects utilizing TRT, which was then compared to the amount of flux penetrating the cow bladder soley from the IllumiCath.
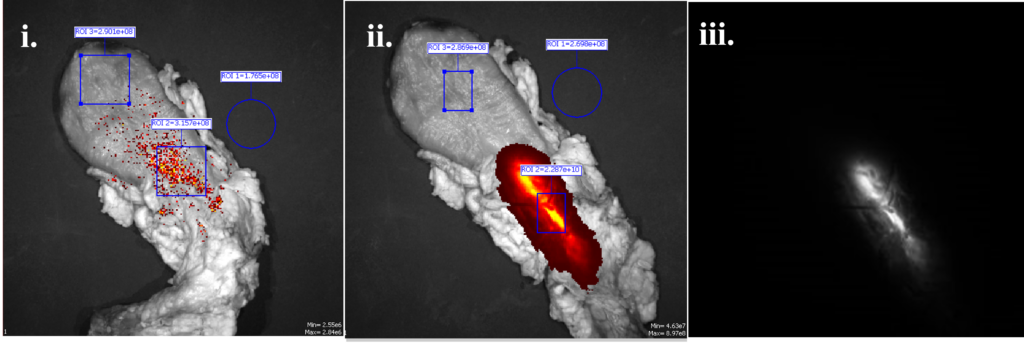
Figure 8: Animal Testing of fluorescence of IllumiCath with IVIS system. The IllumiCath was placed in cow bladder and excited at 745 nm, with an emission filter of 840 nm. Fluorescence of (i) bladder alone (ii) final iteration of IllumiCath in bladder overlayed on bladder. The flux in ROI 2 is 2.287e+10 p/s (iii) IllumiCath fluorescence alone.
Clinical Usage Testing
The Spy-Phi System handheld NIR emitter and camera system by Stryker was utilized to see how the bladder could be visualized with the IllumiCath inside it in a clinical scenario.

Figure 9: W.E.L.D. team using NIR camera-emitter system for the first time to visualize IllumiCath surrounded by bladder tissue. It took the team about 10 minutes to learn how to use the system and record media.
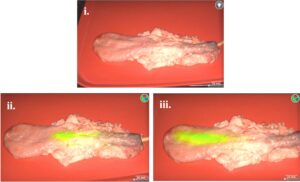
Figure 10: Clinical Testing for IllumiCath Compatibility with the Spy-Phi camera. (i) IllumiCath in the bladder without NIR filter on image (ii) IllumiCath near bottom of bladder (iii) IllumiCath near top of bladder. The length of fluorescence for the IllumiCath is 107 mm.
Regulatory
The IllumiCath is prospectively a Class II device, substantially equivalent to existing devices such as
silicone urinary catheters and the GreenEgg. It would be regulated by 21 CFR 876.5130 for urological catheter and accessories. Therefore, we are pursuing an abbreviated safety and performance based 510(k) pathway submission for FDA clearance.
Financial
Market Size
The IllumiCath makes it possible to see the borders and tissue of the bladder more quickly and precisely, which decreases operating costs from increased operating time as well as additional procedures to treat accidental injuries. Our total addressable market includes all patients that undergo surgeries that utilize Foley catheters, which can be related to the 100,000,000 catheters sold worldwide annually. Our serviceable addressable market consists of the 600,000 hysterectomies that are performed in the United States annually, and our serviceable obtainable market consists of 180,000 hysterectomies performed annually that utilize near-infrared (NIR) technology. The global near-infrared market was valued at 512.67 million USD in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 3.13% from 2024 – 2031, with the fastest growth being observed in devices versus software.
Business Plan for W.E.L.D.
W.E.L.D. plans to evolve its business model as it grows. It will begin with a direct sales model approach, by selling directly to early adopters of the product such as gynecological surgeons who have been performing vaginal hysterectomies for years, or residents currently training in the procedure. Once mass adoption has occurred, our business model will expand to business-to-business, targeting companies that sell NIR devices and software for surgical visualization.
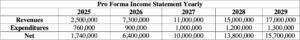
Table 2: Five-Year Pro-Forma Income Statement Table.
Marketing and Sales Strategy
W.E.L.D.’s close ties with Dr. Paula Doyle, a key opinion leader in the field, create a direct pathway for promotion among gynecologic surgeons. W.E.L.D. will target gynecologic surgeons, hospitals, and surgical centers through visually appealing marketing materials and demonstrations that lead to mass adoption of the usage of the IllumiCath, persuading operating room procurement officers to make bulk purchases of the IllumiCath. Through these steps, the IllumiCath can establish itself as the go-to option for all surgeons operating near bladder tissue. W.E.L.D. plans to sell its line of NIR devices and accompanying intellectual property to a larger corporation, preferentially one with an existing customer base in either NIR devices or visualization software.
Reimbursement
According to medicare.gov, the average cost for a vaginal hysterectomy is about $5,548 for the removal of the uterus, fallopian tubes, and ovaries. In comparison, an equivalent laparoscopic hysterectomy costs about $10,030 per procedure.
On average, both Medicare Part A and Part B cover 80% of the costs of medically necessary hysterectomies after deductible expenses have been paid for. Medicare supplement plans also aid in covering hysterectomy costs. There is no extra payment for this procedure if it is a preventative measure.
The IllumiCath would attract insurance companies from its ability to enhance a procedure that is significantly cheaper than alternative routes of treatment through hysterectomy. Its market price of $150 per unit is less than three percent of the total cost of the procedure, and yet would save insurance companies at minimum $3657.60 per laparoscopic hysterectomy replaced with the equivalent vaginal procedure.
Meet the Team!

Judy Monickaraj, Chief Executive Officer, She/Her/Hers

Jazmin Phommavanh, Chief Technical Officer, She/Her/Hers

Aveisha Maharaj, Chief Financial Officer, She/Her/Hers

Acknowledgements
W.E.L.D. would like to thank its partners both within the University of Rochester and beyond for helping to develop this project. We would like to thank Dr. Doyle for her advisement on regulatory, financial, and clinical components of this project, as well as Dr. Wood for his technical advisement and support in testing and prototyping. We would additionally like to thank Martin Gira for his support in planning and the production of our various prototype iterations, and Mahllet Beyene for her contributions to planning Design Day. We would also like to thank Dr. Adamo and Dr. Dirk Stevens for their support of the IllumiCath through the 510(k) pre-submission process. Finally, we would like to thank Brandon Burris, Bob Metzger, Dr. Zavislan, Professor Reidlinger, Erin Sefca, and the Ain Center for Entrepreneurship for their continual support and advisement during business competitions.
References
[1] Hysterectomy. (2022, September 24). Yale Medicine. https://www.yalemedicine.org/conditions/hysterectomy#:~:text=Overview,approximately%20500%2C000%20hysterectomies%20performed%20yearly.
[2] Occhino, J.A. and Gebhart, J.B. (2010) ‘Difficult vaginal hysterectomy,’ Clinical Obstetrics and Gynecology, 53(1), pp. 40–50. https://doi.org/10.1097/grf.0b013e3181ce8945.
[3] Fransvea, P., Chiarello, M. M., Fico, V., Cariati, M., & Brisinda, G. (2024). Indocyanine green: The guide to safer and more effective surgery. World journal of gastrointestinal surgery, 16(3), 641–649. https://doi.org/10.4240/wjgs.v16.i3.641