Team Members
Eric Cecco & Molly Ferris
Supervisors
Greg Gdowski- Executive Director of CMTI Program
Amy Lerner- Academic Director of CMTI Program
Martin Gira- Senior Research Engineer
Clinical Partners
Dr. Eva Galka, Dr. Luke Schoeniger
Description
The MRS is a specimen retrieval device that offers surgeons a way to remove malignant specimens during laparoscopic procedures without significantly widening minimally invasive incisions. The proposed device would use expandable braided sleeving to elongate and radially compress large tissue specimens for extraction through the initial incision. Use of this device would allow surgeons to remove large tissue samples through smaller incisions to reduce patient pain, recovery time, postoperative pain and length of hospital stay.

Unmet Clinical Need
Laparoscopic and robotic surgeries often involve the removal of a tissue sample, which may contain diseased or infectious tissue. When this is the case, an endoscopic tissue removal bag is used to prevent the spread of diseased tissue or infection to the rest of the surgical area or the removal site. However, the removal of larger samples through the ½ inch incisions that are typically made for minimally invasive surgeries is difficult or impossible. This means that surgeons must often widen the incision to as much as 4 inches, compared to open procedures which typically have an incision length of 6 inches. Widening the incision greatly diminishes the benefits of minimally invasive procedures. Bag rupture poses a serious risk to patients as it may lead to the spread of infectious or malignant cells to previously unaffected tissues. There is a growing need for a solution to this problem, as the number of procedures performed using minimally invasive techniques grows rapidly each year.
About this Product
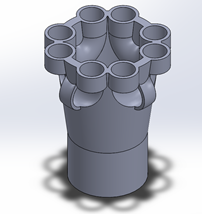
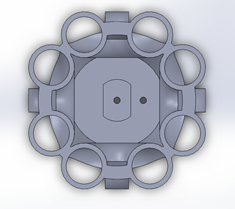
The Minuo Retrieval System uses expandable braided sleeving to elongate and radially compress large tissue specimens for extraction. Inner and outer containment systems ensure that malignant or infectious tissues cannot spread within the body cavity during use. This redundancy provides increased safety and prevents many critical failure modes that are seen in competing products. The streamlined design of the introducing shaft allows for the device to be inserted into the abdominal cavity through a 12mm trocar to minimize any workflow interruptions. A collapsible metal ring at the mouth of the containment system provides an easy and familiar method for loading the tissue specimen. A simplified pulley system and ratcheting mechanism allow the surgeon to extend and remove the tissue with an ergonomic external handle. The distal endcap which allows for this mechanism to function is represented in Figure 1. This device allows surgeons to remove large tissue samples through small incisions used in minimally invasive procedures to reduce patient pain, recovery time, postoperative pain and time in the OR. This device is intended for use in minimally invasive procedures, such as laparoscopic abdominal procedures, where malignant or infectious tissue samples must be removed and pathological analysis may be required. This device is not suggested for use in transplant surgeries. The MRS is a specimen retrieval device that offers hospitals an option to decrease length of stay and potentially increase the volume of patients they can see through decreased incision sizes in most laparoscopic procedures. To launch this product, FDA clearance for a Class II device will be needed through a 510(k) application. This will require bench testing for biocompatibility and efficacy which is currently underway. Clinical testing of this product will not be necessary due to the minimal risk that the device poses to patient health.
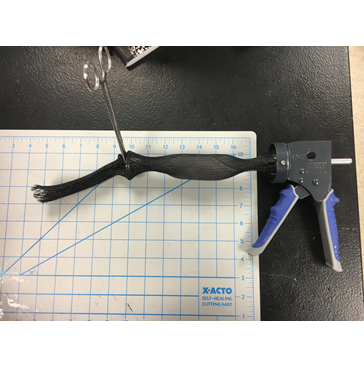
The latest development is the inclusion of a ratcheting mechanism that utilizes a central rod attached to a distal endcap and a rigid attachment of the proximal braided sleeving end to force the distal end away, resulting in radial compression and elongation of the tissue. Figure 2 below shows a rough mockup of the system in action, with some key features missing currently. The endcap has not been fully integrated into the system yet so as a stand-in, a metal plate was used along with a set of hemostats, to secure the distal end of the device. The braided apparatus will also be covered by an additional ripstop nylon bag that has been removed here. The braiding is attached to the ratcheting mechanism with screws that are set when the sleeving is freed from the patient’s abdominal cavity. The central rod is led through the ratcheting mechanism and when the trigger is depressed the mechanism forces the rod to the left, elongating the tissue sample. When fully lengthened, the specimen and all parts of the device can be pulled out of the patient’s abdominal cavity.