Our Team

Daniel Farchione
CMTI Program at University of Rochester (2023)
Biomaterials Engineering at Alfred University (2022)

Justin Jablonski
CMTI Program at University of Rochester (2023)
Biomedical Engineering at University of Rochester (2022)
Abstract
The AortiSeal Ring Securement System was designed to tackle this problem. This device, a pair of interlocking metal rings that hold the valve in place in the heart, is intended to reduce time and material costs of this surgery, lead to more consistent sealing of the prosthetic valves, and will allow for easier surgical revision without compromising the integrity of the implant. Our device aims to facilitate surgical aortic valve replacements to benefit both surgeons and patients.
Aortic Valve Replacements
Surgical aortic valve replacements (sAVRs) are commonly used to treat aortic valve stenosis, a severe condition in which the heart valves calcify and stiffen, restricting blood flow through the body. This can lead to complications such as blood clots, stroke, heart failure, and arrhythmias, and untreated stenosis has a 50% mortality rate over a 2-year period. During sAVRs, the aortic valve is removed and a prosthesis is attached to the aortic annulus via suturing, and this manual attachment process can lead to uneven suture spacing and tangling of sutures. Slow suture placement also extends the time the patient must be on heart-lung bypass, significantly increasing their risk of complications1,2. The video below goes through the process of a typical sAVR procedure, which further highlights the key steps involved for successful suturing.
Additionally, for patients who undergo the surgery when young and must get revision replacements with new valves as they age, tissue integration of the sutures complicates the dissection of the valve for secondary replacements.
The AortiSeal
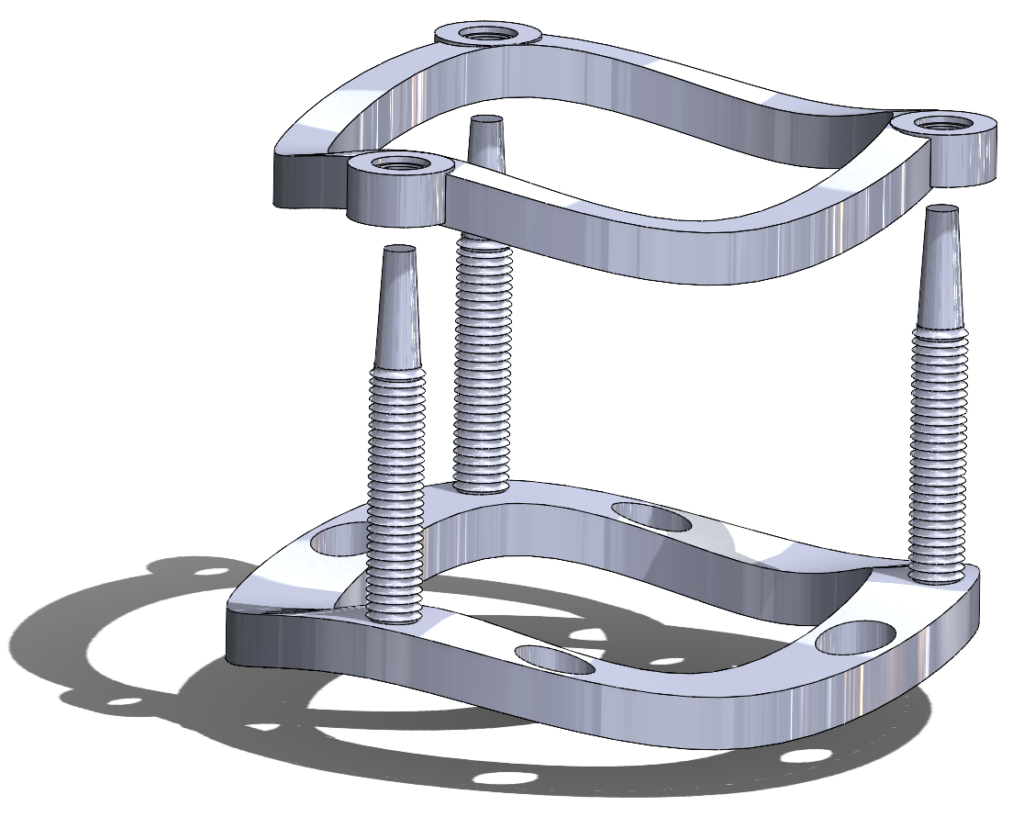
The AortiSeal Ring Securement System is a device designed to rapidly secure an aortic valve to the annulus tissue during a surgical aortic valve replacement, consisting of a pair of implanted locking rings. These locking rings would include a base ring, which could be attached to the heart with a single continuous suture, and a securement ring, which would lock into the base ring, with the sewing cuff of the prosthetic valve sandwiched between the two rings. This locking mechanism would be via screws, giving a reversible mechanism that allows for easy removal of the top ring and the prosthetic valve in cases of surgical revision or future valve replacements.
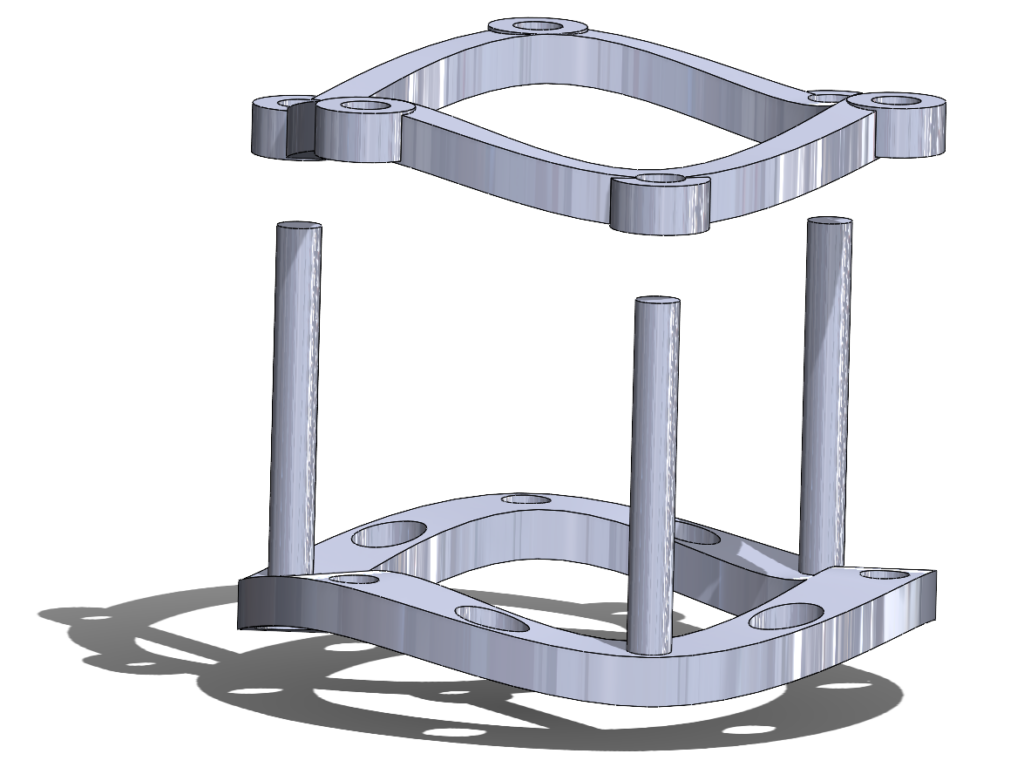
Figure 5. A 3D rendering of the AortiSeal Ring Securement System. The device is composed of a basal ring with guideposts and a securement ring, which lock together to secure a prosthetic valve between the two rings.
This device has several advantages when compared to a traditional surgical aortic valve replacement, with the primary benefits related to the time and effort saved in the surgery. We expect our device to significantly reduce the time taken to implant the prosthetic valve, reducing time on heart-lung bypass to reduce patient complications and overall operating time. Additionally, the Ring Securement System will reduce the material costs during the surgery, as it requires only a single suture to implant and thus reduces the number of sutures and the complexity of the suturing required. This simplicity of implanting will also increase consistency across different surgeries. Finally, the device allows for retrievability of the prosthetic valve, as the locking mechanism (the screws) can be released to easily remove the valve. This will allow for less dissection and local damage to tissue during revision surgeries, reducing complications for patients in future surgeries as well.
Currently, this device is still within the prototype stage, and will require further iterations to reach a clinically testable stage. These iterations primarily revolve around the material choice of the device, the design of an applicator tool to facilitate in the screw securement of the rings, and the scaling of the device to fit multiple valve diameters.
Our Device: Evolution
Initial Conceptual Prototype
We first came up with the concept of a pair of interlocking rings in February 2023, while working on developing a device to facilitate suture placement. This idea stemmed from the initial idea of a ‘guide ring’ to help with suture placement, which we realized would be too difficult to remove after use. However, this inspired the idea of developing a ring that stayed in place in the heart after surgery. The top ring then followed suit as a way to make the securement of the valve to the bottom guide ring faster, and thus our device was born.
Our initial device included a series of pins that protruded from the top securement ring, which would push through the sewing cuff of a prosthetic valve and lock into the bottom ring, providing both a firm hold on the valve and attachment of the two rings together.
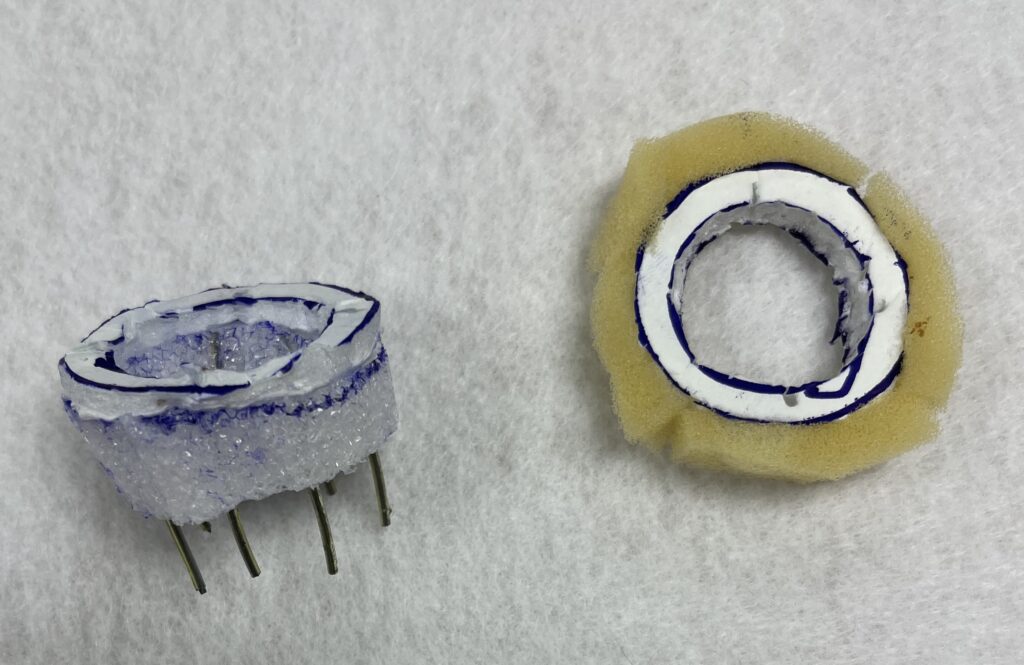
1st Iteration Prototype – Locking Pins
Our first prototype iterations focused on capturing the geometries of prosthetic valves, especially the curved nature of the valve sewing cuffs. As we further developed this concept, we decided to forego the large number of locking pins, primarily due to difficulties in manufacture of the pins but also due to the difficulty of aligning these pins correctly when placing the valve. This next prototype was much easier to 3D print and could more easily be placed around a valve, but lacked a locking mechanism to hold the rings together.
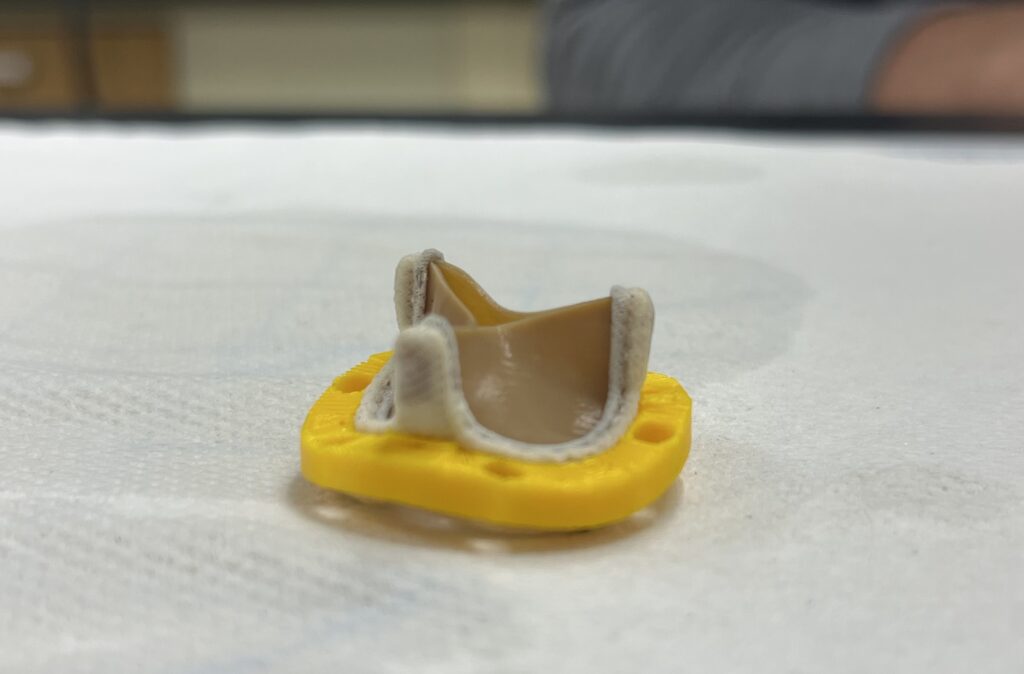
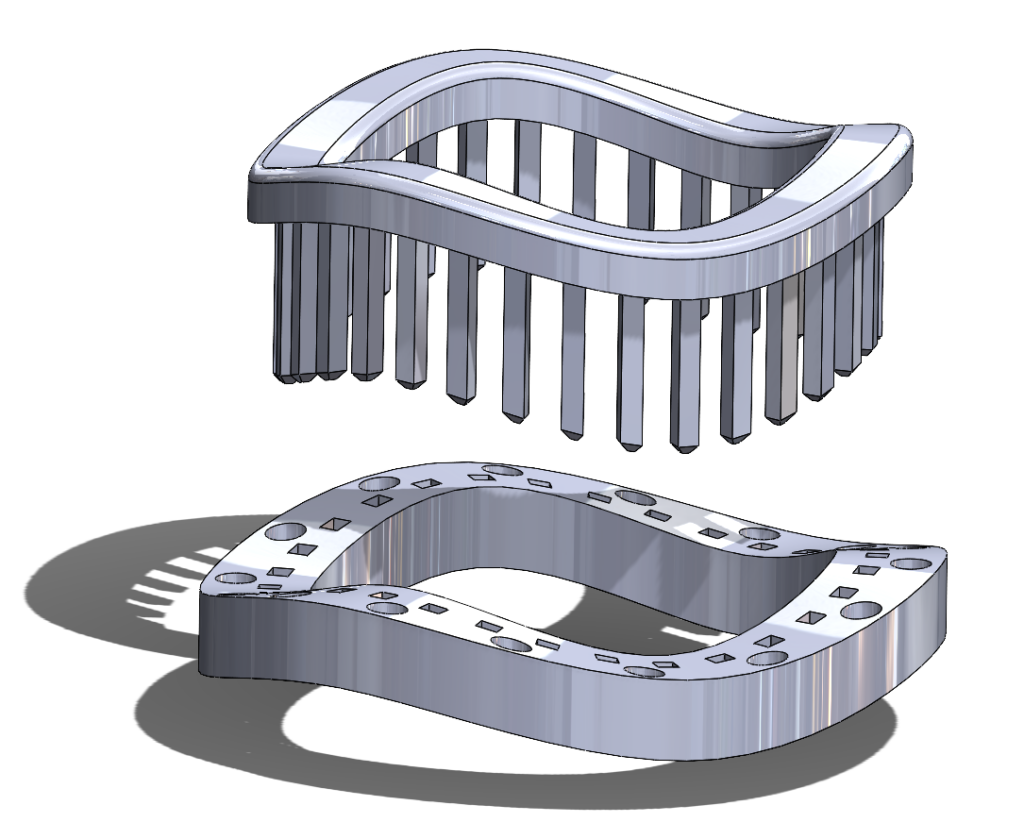
2nd Iteration Prototype – ‘Ziptie’ Mechanism
Our next prototypes needed to add a method to secure the two rings together, with the goal of holding the prosthetic valve clamped between the two rings. We could then verify if the additional pins present in our original concept were necessary to keep the valve secured between the rings, or if the compression force between the two rings was sufficient to hold the suturing cuff steady.
We decided to try a ‘ziptie’-like method of securing the rings, with a one-way locking mechanism to secure and tighten the rings. This required the building of guideposts that would hold the ‘ziptie’ barbs.
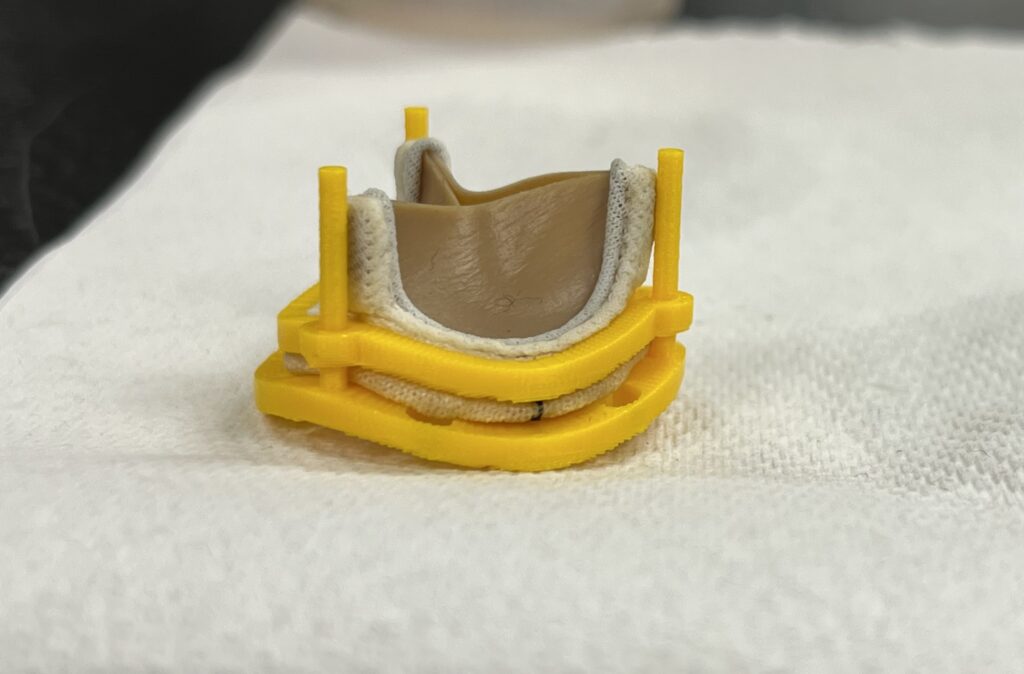
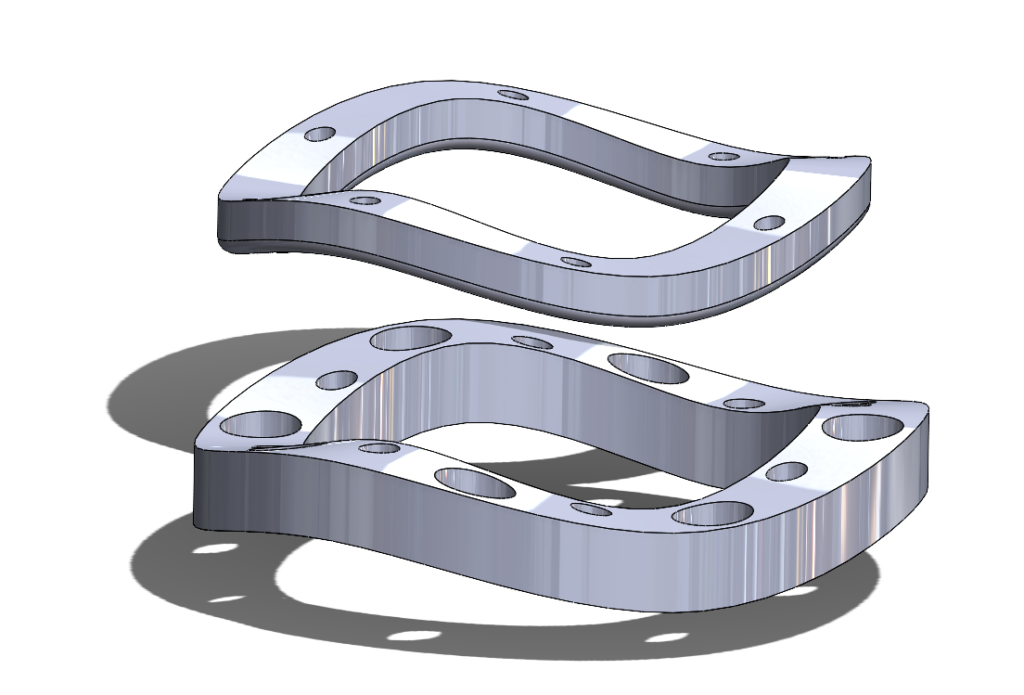
3rd Iteration Prototype – Screw-Based Locking
The ‘Ziptie’ mechanisms in our previous prototype neither printed well nor held together well, and were another irreversible locking mechanism, and so we decided to move away from this direction. The guideposts, however, were useful in keeping the rings aligned and so remained in our design.
We were then given the idea of using small screws to hold the two rings together. While this may ultimately require development of an additional application device to facilitate the screwing in a small environment, use of screws would allow for a strong but easily-reversible method of attaching the rings together. Additionally, the simpler geometries of screw holes would print much better than the more complex securement methods we previously had. After designing and testing a pair of locking rings using screws, we ultimately decided that this would be the best method available to us for securing the two rings together in a strong but reversible seal.
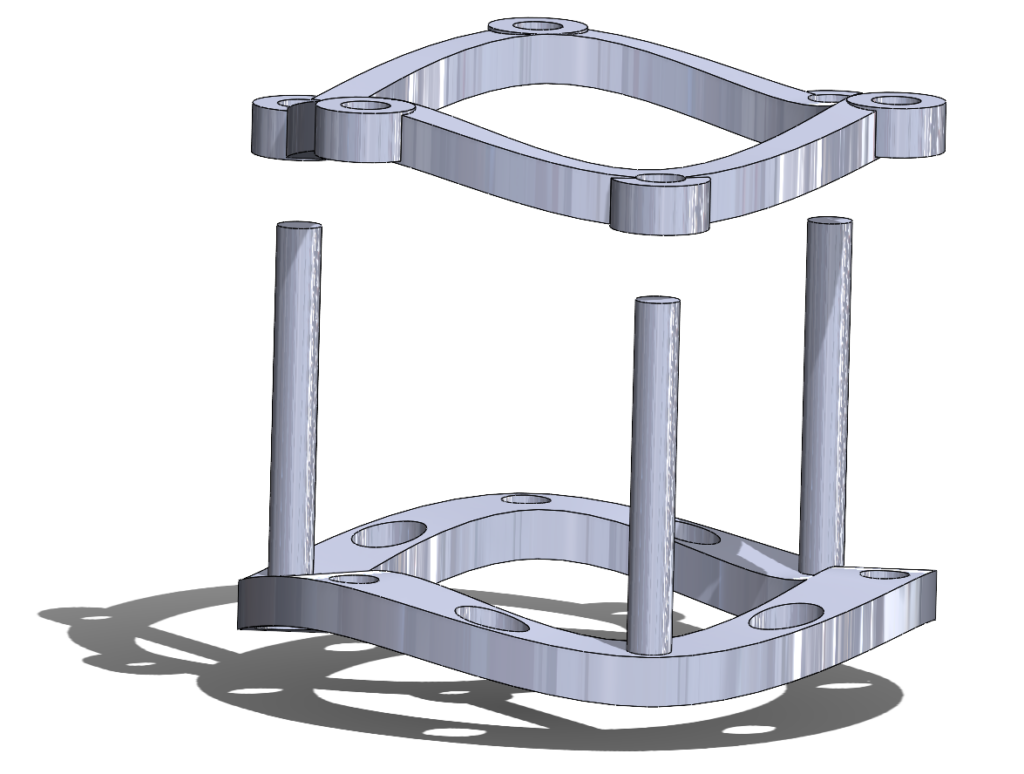

Our Device: Marketability
Our device, the AortiSeal Ring Securement System, is a device designed for use within open and minimally-invasive aortic valve replacement surgeries. This market for surgical aortic valve replacements (sAVRs) is a respectable size, with over 50,000 patients receiving open-heart aortic valve replacements yearly in the US alone and around 280,000 sAVRs globally.3
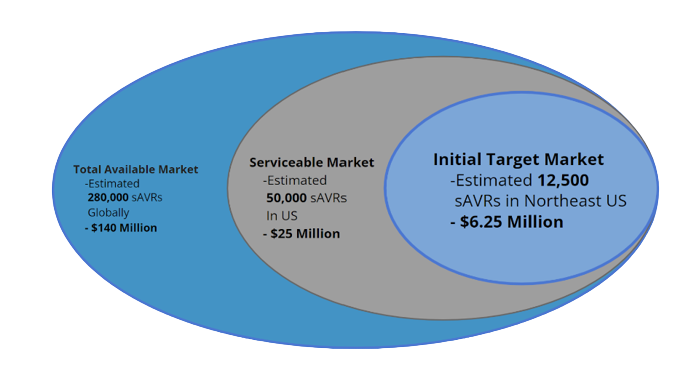
The top current competitors in this market include suture placement devices and expanding surgical valves. Current suture placement devices do not address the number of sutures placed or the time required to do so, whereas expanding valves that deploy faster have higher risks of complications due to expansion providing a poorer attachment of the valve in the heart. There are not any currently-marketed devices that address both the time and organization issues required to place sutures and the quality of the valve seal formed, creating an opportunity for a device that can improve all of these issues. Additionally, the novelty of a device that allows for easy removal of the prosthetic valve in cases of future revisions further adds to the value of our device.
Our Device: Regulation
The AortiSeal Ring Securement System is a novel implantable device. Conversations with FDA officials confirmed that there are neither predicate devices nor applicable product codes for this system, meaning that an entire new product code could be established if our device is approved.
Due to the high-risk nature of our device as a blood-contacting implant, our device will require a PMA submission with extensive clinical testing for FDA review and approval. We have developed a battery of potential preclinical tests that can be pursued to demonstrate the functionality of our device and assist in development of a PMA.
Our Device: Testing
We performed testing on our system while it was clamped around a prosthetic valve, to determine if the valve would slip out of the ring assembly and to discover what the leak rate through the clamped seal would be. Our goal was to demonstrate that the rings could securely hold the valve without compromising the seal the valve would have, making attachment of the prosthetic with the AortiSeal functionally equivalent to attachment of the prosthetic with normal suturing.
To test this, the following setups were used. We had an acrylic column in which water could be placed, with the base ring of our device glued to the bottom and the valve and top ring screwed on. This allowed for direct measurements of the leak through the seal, albeit with a slightly lower pressure than would be experienced under anatomic conditions.

We also developed a smaller pressurized system we could use to test the valve leakage under pressures closer to normal blood pressures (80-120 mmHg). This system composed a chamber with a pressure valve attached and a syringe, which was used to pressurize the system. The valve was again secured to the bottom of the chamber, with the base ring glued into the chamber to provide a watertight seal around the base.
Neither system showed any signs of valve slippage or repositioning, demonstrating that compression of the valve suturing cuff between the two rings was sufficient to hold the valve in place.

The pressurized system unfortunately had several leaks that occurred between the glue seals due to poor adhesion to the plastic, however from the acrylic column system we were able to get several leakage rate measurements. We also attempted to use this system with a cap over the valve (pictured below) to remove any leakage occurring through the valve, however the geometry of this cap held the valves in a warped position that interfered with the suturing cuffs and actually increased the leakage through the seal.
We measured leak rates of 1.9 ± 0.6 mL/min without the cap and 3.9 ± 0.2 mL/min with the cap occurring through the system. While we were initially worried that this would be high, literature on paravalvular leakage rates mentioned that leakage rates under 30 mL/beat were considered mild and did not require clinical intervention. This rate falls to nearly 1.8 L/min for an average heartrate, almost 1,000 times higher than our leak rate through the seal4. Additionally, since our system was tested under static pressure and not the dynamic flow of a real heart, the frequent outflow through the valve may further reduce reverse leakage through the seal. Thus, our test successfully demonstrated a very low leak rate through the seal that would not be clinically significant.

Acknowledgments
Special thanks to: Greg Gdowski, PhD, Martin Gira, Jonathon Stone, MD, Peter Knight, MD
References
1. Ancona, R., Pinto, S. (2020) Epidemiology of aortic valve stenosis (AS) and of aortic valve incompetence (AI): is the prevalence of AS/AI similar in different parts of the world? Escardio.org. Acc. 20 Apr. 2023.
2. Cedars-Sinai (2020). NEJM: Transcatheter Aortic Valve Replacement Shows Similar Safety Outcomes as Open-Heart Surgery. Cedars-Sinai.org, Acc. 20 Apr. 2023.
3. Global Transformational Health Research Team at Frost & Sullivan (2021). Global Structural Heart Devices Growth Opportunities. Frost & Sullivan, Acc. 20 Apr. 2023.
4. Lerakis, S., Hayek, S. S., & Douglas, P. S. (2013). Paravalvular aortic leak after transcatheter aortic valve replacement: current knowledge. Circulation, 127(3), 397-407. Acc. 20 Apr. 2023
