Design Team





Customer

Tim Adolf, Benjamin Boseck, Matthew DeClerck, Sandra Madanat, Simbi Maphosa, and John Moyer
Problem Statement
A cardiac phantom model has been developed as an experimental and demonstrative tool for left atrial appendage (LAA) occlusion procedures. In patients with non-valvular atrial fibrillation, blood flow stagnation in the LAA, a small protrusion extending from the left atrium, can induce clot formation and subsequent stroke. The heart phantom offers a platform to evaluate the efficacy of occlusion devices and features highly-regulated pulsatile flow, the ability to modulate the base diameter and wall thickness of the LAA, and visualization of flow dynamics within the appendage.
Background
Atrial fibrillation (AF) is a cardiac arrhythmia characterized by irregular electrical stimulation of the atria [1]. The disorder has a diverse range of symptoms and pathological effects, and is a result of the development of abnormalities in the atria’s electrophysiological properties, structural irregularities, or a combination of both [2-4]. AF can manifest itself in the form of palpitations, shortness of breath, and fatigue, leading to a decreased quality of life for those afflicted [1]. Further, AF is associated with a high risk of clot formation and subsequent stroke incidence [1].
Non-valvular atrial fibrillation is a specific subset of this condition in which valvular disease is absent [2]. The precise etiology of non-valvular AF remains an area of active research; however, it has been established that the disorder likely arises from complex interactions of genetic, environmental, and lifestyle factors [2].
Though treatment options range from pharmacotherapeutics to surgical procedures, the most common therapeutic modality is anticoagulant treatment [2-4]. Blood thinners can reduce the risk of stroke by preventing thrombus formation, though some patients may not be suitable candidates for long-term anticoagulation due to factors such as age, high risk of bleeding, or limited response to these medications [4]. In fact, it has been estimated that only approximately 60% of patients on these therapeutics maintained effective anticoagulant protection. Further, a life-long dependence on medication can be financially and psychologically burdening to many patients [5].
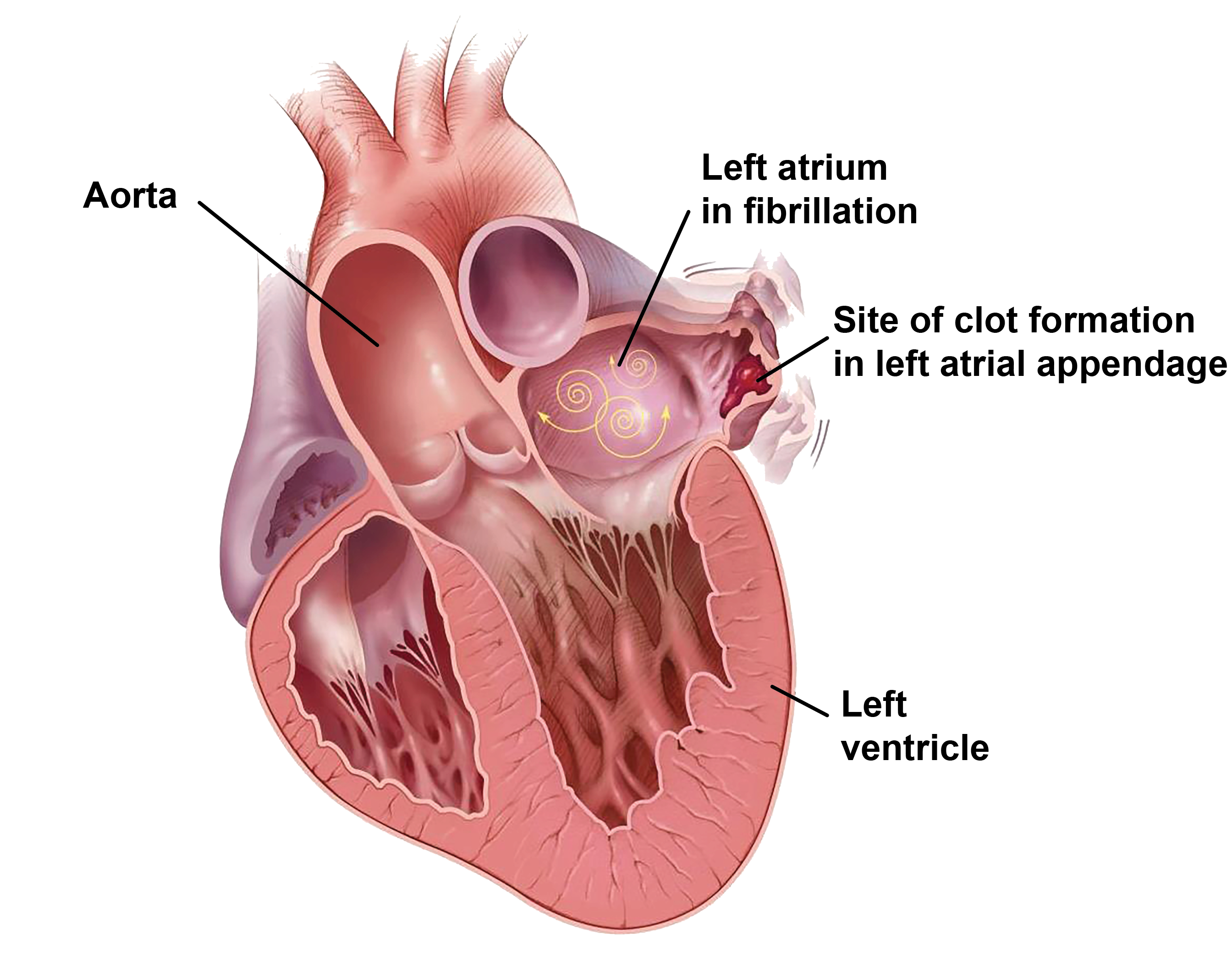
The left atrial appendage (LAA) is a finger-like, trabecular protrusion extending off the left atrium [5-6]. In patients with non-valvular AF, blood flow stagnation within the LAA can result in thrombus formation. In fact, 90% of clots in patients with non-rheumatic atrial fibrillation are located within the LAA [5-9].
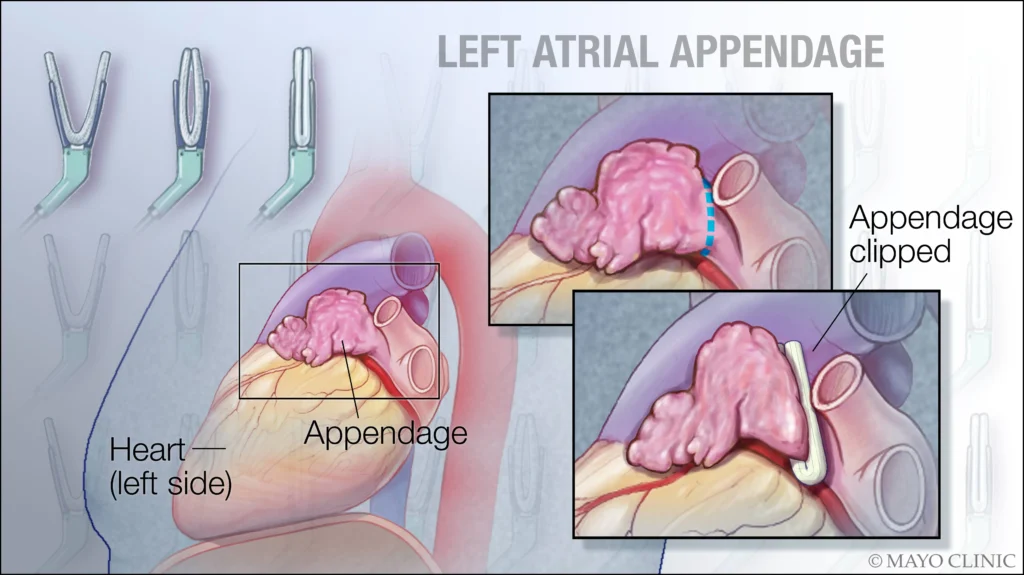
Procedural occlusion of the appendage can limit blood flow into and out of the LAA, preventing clot formation and/or propagation [8-10].
Design Specifications
This project has focused on the development of a cardiac phantom model for use as a framework for testing and demonstration of LAA occlusion techniques. The device is used as a testbed for the investigation and refinement of appendage occlusion procedures. Thus, it was critical that the device design considered the biomechanical properties of cardiac tissue and the physiological flow characteristics of the heart. Establishing a phantom model analogous to the heart, particularly with respect to the LAA, would effectively function as a demonstrative tool, enable realistic surgical training, and serve to validate the efficacy of occlusion procedures.
| Metrics | Goal |
|---|---|
| Appendage dimensions are physiologically relevant | Appendage length of 20-40 mm, volume of 2-4 mL, and wall thickness of 1.5-3 mm [11-12] |
| Biomechanical accuracy of LAA components | Material used reflects elastic modulus of heart tissue (40-70 kPa), as well as compliance (~0.1 cm3/mmHg) [13-14] |
| Modularity of LAAs, allowing for multiple geometries/replicates to be tested | Modules can be replaced easily, and all connection interfaces are leak-free |
| Pulsatile flow of blood facsimile | Flow is pulsatile and reflects physiological pressure and volume characteristics |
| Appendage occlusion efficacy indicated | Ability to visualize/quantify flow into appendage following simulated occlusion |
Overall System Schematic

Material Selection
Ecoflex™ 00-30, a two-part silicone elastomer, was selected for its durability, translucency, and resemblance to native cardiac tissue [13-14]. Its shore hardness translates to an elastic modulus of 68.9 kPa, and the material compliance of a hollow silicone shell of a general appendage geometry was experimentally determined to be 0.11 cm3/mmHg. This silicone resin was injected into custom-designed molds to cast LAA modules.
Manufacturing Workflow: Mold-Box Design, LAA Casting, and Base Atrium Frame

After a raw MRI scan of a patient’s heart was sourced, a series of boolean mesh expressions was applied to isolate the left atrial appendage, as indicated above. All abrupt, sharp edges on the appendage were smoothed using the open-source software Blender 4.0. It was then possible to re-mesh all surfaces for a simplified, but detailed, mesh configuration of the appendage. The geometry of the LAA was then anthropometrically verified, with the orifice diameter at 21 mm and the wall thickness at 2 mm, matching physiological dimensions [11-12]. To reflect the heterogeneity of the LAA geometry among the patient population, the sourced appendage was scaled to develop three components of various sizes encompassing the standard range of dimensions.
Next, a three-piece mold box, for the casting of LAA modules, was developed using SolidWorks CAD software. A three-piece set was selected to ease the demolding process, and a mold-box was designed for each of the three appendage sizes considered. Mating features included a rectangular insert and a coupling rim around the circumference of the box. Several vent holes were placed at the highest points of the appendage geometry to allow for air to escape. An injection site, compatible with standard syringes, was included. Lastly, peg-like features on the bottom piece were used to cast through-holes in the appendage membrane for attachment to other device components. All mold-boxes were 3D printed with polylactic acid filament using fused deposition modeling technology. To cast LAA components, Ecoflex™ 00-30 was mixed, degassed at 27 psi vacuum, injected into the mold-box, and allowed to cure.

Lastly, a base frame resembling the heart’s left atrium was designed using SolidWorks. A connection interface matching the through-holes in the LAA casts was included on the top face of the frame, along with ribbed connectors for tubing attachment. This frame was stereolithographically printed in a clear resin. For device assembly, the modular appendage component was placed on top of the base frame, with M3 bolts guided through the pre-cast holes, and a 3D-printed ring washer plate was positioned to apply a uniform pressure distribution along the cast piece.

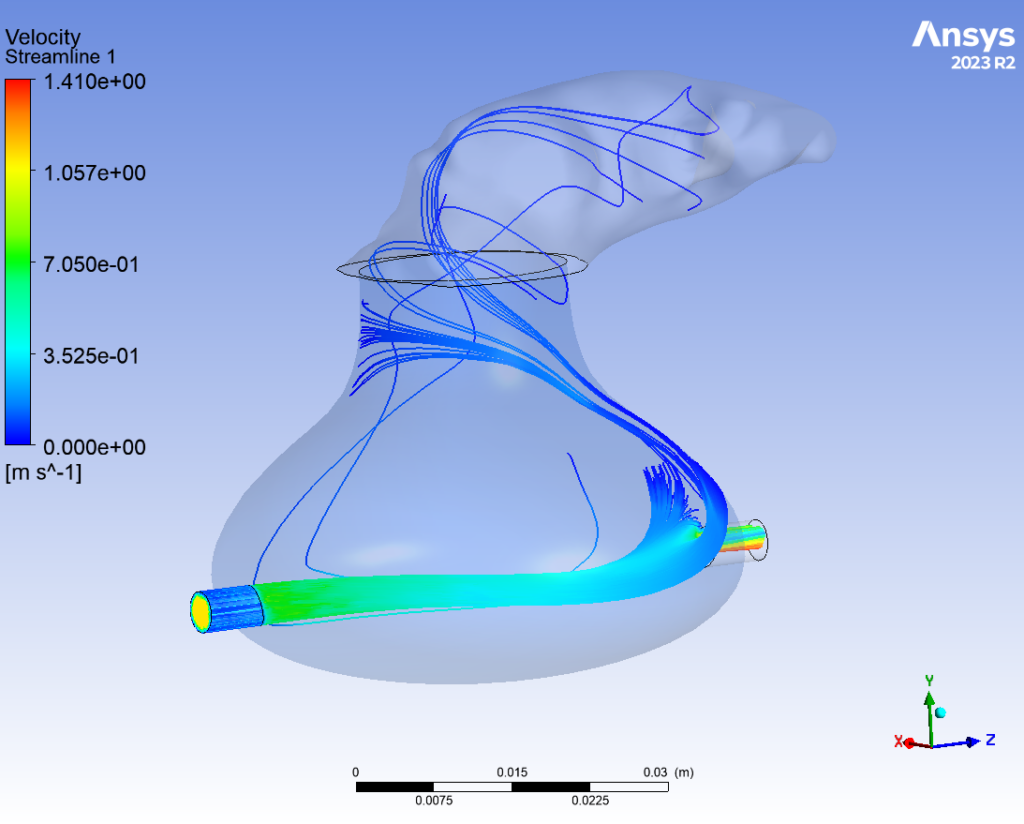
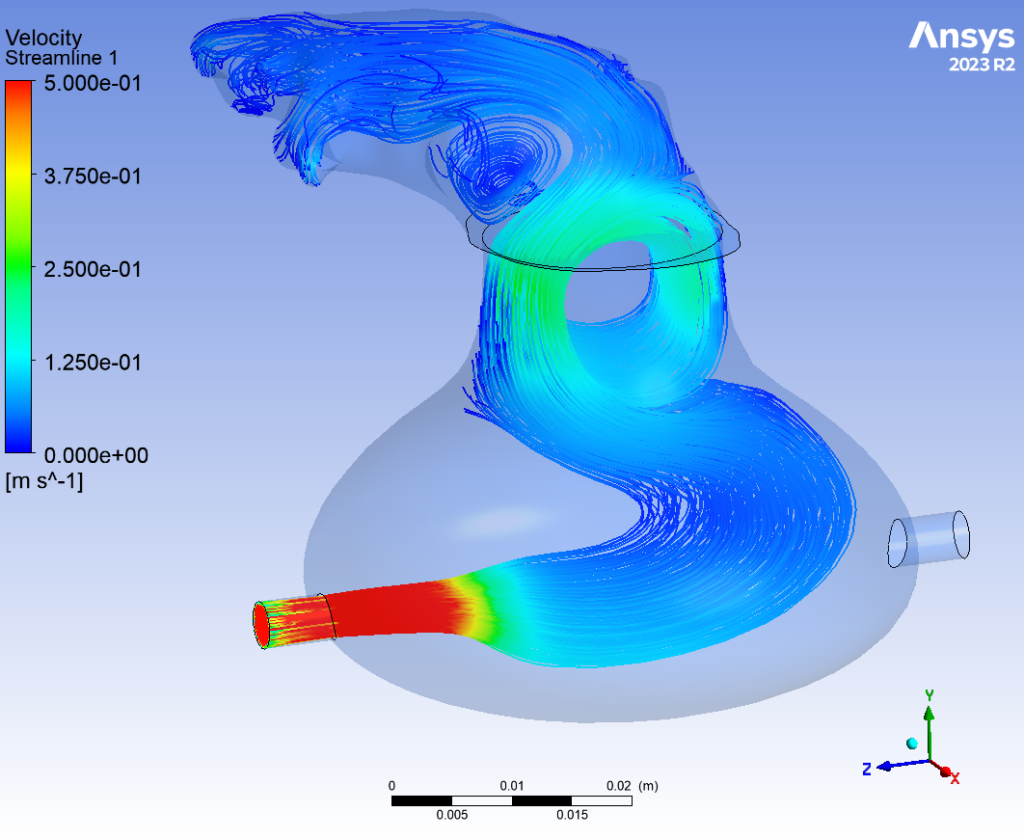
Flow Dynamics
The behavior of flow through the appendage was simulated computationally using ANSYS Fluent. This finite element analysis was performed under two conditions: one with the outlet valve open, and the other while closed. When the valve blocked the flow outlet, fluid was able to accumulate in the LAA, building pressure within the device. When the valve opened, fluid effectively drained from the appendage. This analysis validated the proposed fluid system; by controlling the flow rate of the peristaltic pump and the timing of the valve closure, a “heart-beat” could effectively be simulated.
Device Function and User Interface
All components of the device, including the pressure sensor, solenoid valve, peristaltic pump, and user inputs, are controlled using an Arduino R3 microcontroller. The software program receives input from the user and pressure sensor to modulate the flow rate of the peristaltic pump and the state of the solenoid valve in order to simulate the function of a heart. Once the pulsatile flow has been established, the LAA occlusion technique of focus can be applied, and the efficacy of flow obstruction evaluated by visualizing the extent to which fluid can circulate into the appendage.

Future Directions
- Designing additional mold-boxes with various appendage morphologies, sizes, wall thicknesses, base diameters, etc.
- Streamlining the current manufacturing workflow, so that a raw scan of any patient’s LAA can be efficiently cast to serve as training and demonstrative tool
- Validating the efficacy of an occlusion device on a patient-specific geometry
Acknowledgements
We would like to thank LSI Solutions for the opportunity to contribute to this project. We would also like to thank our course professor, Dr. Scott Seidman, our faculty supervisor, Dr. James McGrath, the senior laboratory engineer, Martin Gira, and the fabrication shop manager, Jim Alkins, for aiding us throughout the process.
References
[1] S. Nattel, “New ideas about atrial fibrillation 50 years on,” Nature, vol. 415, no. 6868, Art. no. 6868, Jan. 2002, doi: 10.1038/415219a.
[2] E. L. C. Pritchett, “Management of Atrial Fibrillation,” N. Engl. J. Med., vol. 326, no. 19, pp. 1264–1271, May 1992, doi: 10.1056/NEJM199205073261906.
[3] E. N. Prystowsky, B. J. Padanilam, and R. I. Fogel, “Treatment of Atrial Fibrillation,” JAMA, vol. 314, no. 3, pp. 278–288, Jul. 2015, doi: 10.1001/jama.2015.7505.
[4] C. Pappone and V. Santinelli, “Atrial Fibrillation Ablation: State of the Art,” Am. J. Cardiol., vol. 96, no. 12, Supplement 1, pp. 59–64, Dec. 2005, doi: 10.1016/j.amjcard.2005.09.063.
[5] K. Bedeir, D. R. Holmes, J. L. Cox, and B. Ramlawi, “Left atrial appendage exclusion: An alternative to anticoagulation in nonvalvular atrial fibrillation,” J. Thorac. Cardiovasc. Surg., vol. 153, no. 5, pp. 1097–1105, May 2017, doi: 10.1016/j.jtcvs.2016.12.040.
[6] R. Beigel, N. C. Wunderlich, S. Y. Ho, R. Arsanjani, and R. J. Siegel, “The left atrial appendage: anatomy, function, and noninvasive evaluation,” JACC Cardiovasc. Imaging, vol. 7, no. 12, pp. 1251–1265, Dec. 2014, doi: 10.1016/j.jcmg.2014.08.009.
[7] N. M. Al-Saady, O. A. Obel, and A. J. Camm, “Left atrial appendage: structure, function, and role in thromboembolism,” Heart, vol. 82, no. 5, pp. 547–554, Nov. 1999, doi: 10.1136/hrt.82.5.547.
[8] J. J. Squiers and J. R. Edgerton, “Surgical Closure of the Left Atrial Appendage: The Past, The Present, The Future,” J. Atr. Fibrillation, vol. 10, no. 5, p. 1642, Feb. 2018, doi: 10.4022/jafib.1642.
[9] K. Ramoju, “The Left atrial appendage closure” pp. 165–169, Jan. 2016.
[10] E. Zimmermann, “To have or not to have…your left atrial appendage closed,” Mayo Clinic News Network. Accessed: May 01, 2024. [Online]. Available: https://newsnetwork.mayoclinic.org/discussion/to-have-or-not-to-haveyour-left-atrial-appendage-closed/
[11] K. Słodowska et al., “Morphology of the Left Atrial Appendage: Introduction of a New Simplified Shape-Based Classification System,” Heart Lung Circ., vol. 30, no. 7, pp. 1014–1022, Jul. 2021, doi: 10.1016/j.hlc.2020.12.006.
[12] K. Słodowska et al., “Thickness of the left atrial wall surrounding the left atrial appendage orifice,” J. Cardiovasc. Electrophysiol., vol. 32, no. 8, pp. 2262–2268, 2021, doi: 10.1111/jce.15157.
[13] Y. Takaya et al., “Left atrial appendage morphology with the progression of atrial fibrillation,” PLOS ONE, vol. 17, no. 11, p. e0278172, Nov. 2022, doi: 10.1371/journal.pone.0278172.
[14] C. A. Davis, J. C. Rembert, and J. C. Greenfield, “Compliance of left atrium with and without left atrium appendage,” Am. J. Physiol.-Heart Circ. Physiol., vol. 259, no. 4, pp. H1006–H1008, Oct. 1990, doi: 10.1152/ajpheart.1990.259.4.H1006.
[15] “Newsfeed,” Sketchfab. Accessed: May 01, 2024. [Online]. Available: https://sketchfab.com/3d-models/adult-blood-volume-heart-model-heart0131-511946cc99ce4d0a9795f7cd8555de19
