Team and Specialty
We are Heart Smart, a cardiovascular surgery team from the CMTI program at the University of Rochester. Our team is composed of Katherine Gregory, Mitchell Hoestermann, and Andrew Hirsh.



Medical Condition
The aortic valve prevents backflow between the aorta and left ventricle of the heart. Two diseases that lead to an aortic valve replacement surgery are aortic valve regurgitation and aortic valve stenosis. Aortic valve regurgitation occurs when blood flows back into the heart, which can be caused by the deterioration of leaflets, abnormal valve shape (bicuspid instead of tricuspid), or bacterial infection [1]. Aortic valve stenosis occurs when the aortic valve is narrowed or obstructed by calcification causing the valve to harden, leading to less blood pumping into the aorta per heartbeat [1].

Surgical Difficulty/Problem
One of the largest risks when conducting an aortic valve replacement (AVR) is the possibility of a calcium embolization to occur post procedure. During the debridement process, small calcium particles are capable of breaking off and falling into the left ventricle of the patient [2]. These particles can act as a seed for future calcification to occur and are able to spread throughout the patient’s vasculature. The locations in which these emboli are found are in both minor and major coronary arteries with an occurrence of 11% and 6% in cases, respectively [3]. Current methods used to collect this debris are the placement of either gauze or sponges and irrigation of the left ventricle with saline solution.
Market Opportunity
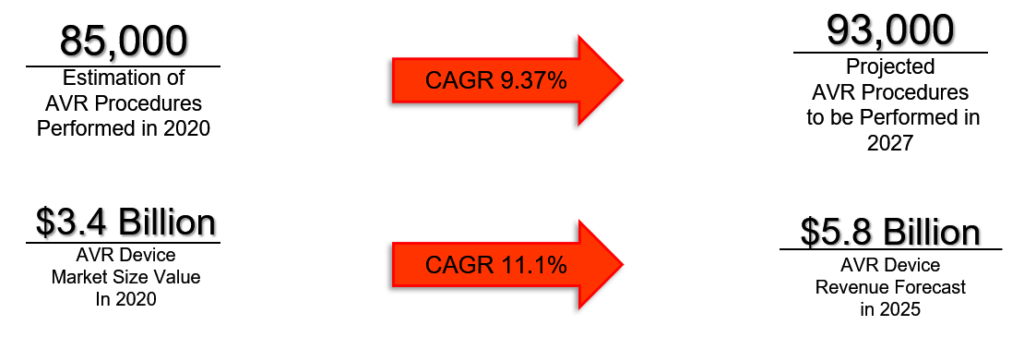
The Calcium Catcher is specifically designed to be used in procedures for patients undergoing an AVR. Patients that have either regurgitation or stenosis are eligible for an AVR. This patient segment makes up 65.2% of all valvular disease patients, or 16.9 million patients in total. We are focusing on patients with valve degradation through calcification, and 44.9% of the 16.9 million valvular disease patients fall within this specific group [4]. Our target market size has 7.4 million potential surgeries in which our technology can be utilized. There were 85,000 AVR procedures performed in 2020 and this surgery is expected to grow to 93,000 procedures at a compound annual growth rate (CAGR) of 9.37% from 2020-2027 [5]. The AVR device market was valued at $3.2 billion in 2020 and is expected to grow at a CAGR of 11.1% through 2025 [6].
BioDesign Statement
A device for cardiac surgeons to minimize emboli caused by calcium debris dislodged while taking out the aortic valve during aortic valve replacement surgeries.
Major Design Requirements
When conducting Voice of Customer (V.o.C.) meetings, we learned about specific requirements needed for our device. These requirements included: a removable handle to allow proper visualization of the region of operation during surgery, a limited amount of force applied to the wall of the left ventricle to prevent heart block, and to make sure the device produces a flush surface with the vessel walls. Besides these needs required by the customer, the team came up with several other requirements such as the device is water-resistant and permeable, capable of maintaining its shape, and can capture debris produced during the surgery.
Prototypes

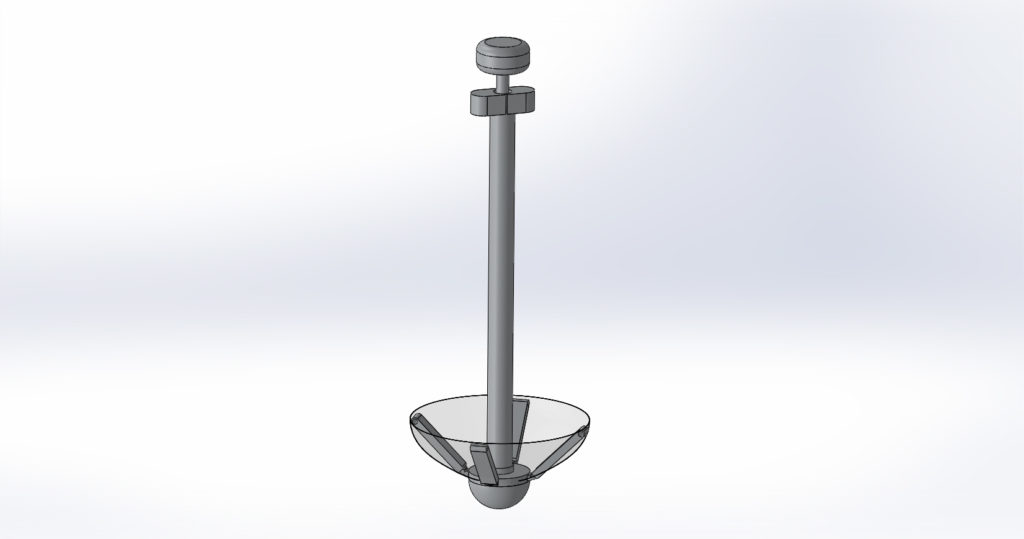
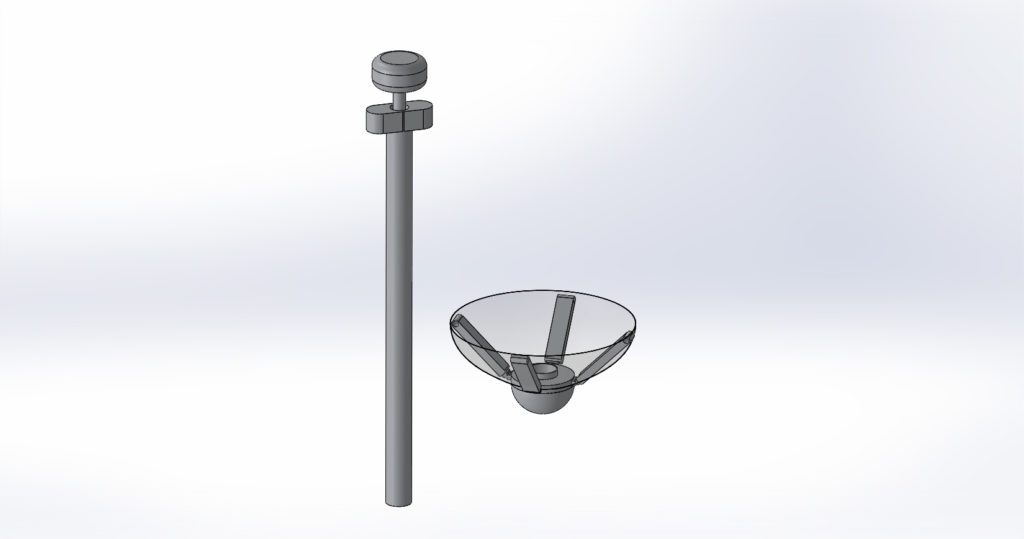
The final prototype displayed above is of the 4x scale model of the Calcium Catcher. The initial image shown is of the 3D printed body in full assembly which includes: a polyester mesh, a handle, arms, deployment mechanism and a retrieval mechanism. Also shown are the CAD models that were used to 3D print this model.
Testing
We plan on testing the device against the surgical sponge that is currently used in AVRs. To test effectiveness, we will deposit calcium carbonate debris into the filter and irrigate with 100mL of saline. We will deposit the same amount of calcium carbonate on the surgical sponge and irrigate the area with 100mL. We will use a cavity that will be the size of the aorta or a scaled up version to capture the liquid. We will do a gross examination of the liquid samples to see which one performed best. We also will do studies on ex-vivo porcine hearts to look at the safety of our device.
Regulatory Strategy
We are proposing to market our device as a Class II device through the 510(k) pathway. Our device requires general and special controls. We met with a FDA reviewer to discuss our mock Pre-Submission, and we determined that our device should have the product code PUM and be regulated under the Cardiovascular medical specialty panel.
Conclusions
The Calcium Catcher by Heart Smart is a medical device for cardiac surgeons who perform aortic valve replacement surgeries and have a problem with calcium debris dislodging from the aortic valve during removal. Our product provides a method to capture calcium debris in order to limit stroke incidence. Unlike currently used surgical sponges, the advantage of our product is that it is more efficient at catching debris.
Acknowledgments
Heart Smart would like to thank Dr. Greg Gdowski, Martin Gira, Dr. Peter Knight, and Michael Pyszczek and Aliza Panjwani for their academic, engineering, clinical, and business support of our project.
References
[1] Aortic valve repair and aortic valve replacement. (2020, August 07). Retrieved September 06, 2020, from https://www.mayoclinic.org/tests-procedures/aortic-valve-repair-aortic-valve-replacement/about/pac-20385093
[2] Neri, E., Toscano, T., Frati, G., Capannini, G., Bizzarri, F., & Sassi, C. (2001). A simple method to prevent calcium embolization during aortic valve surgery. Texas Heart Institute journal, 28(4), 320–321.
[3] Holley, K. E., Bahn, R. C., Mcgoon, D. C., & Mankin, H. T. (1963). Spontaneous Calcific Embolization Associated with Calcific Aortic Stenosis. Circulation,27(2), 197-202. doi:10.1161/01.cir.27.2.197
[4] Bartus, K., Sadowski, J., Litwinowicz, R., Filip, G., Jasinski, M., Deja, M., Kusmierczyk, M., Pawlak, S., Jemielity, M., Jagielak, D., Hendzel, P., Suwalski, P., Tobota, Z., Maruszewski, B., & Kapelak, B. (2019). Changing trends in aortic valve procedures over the past ten years-from mechanical prosthesis via stented bioprosthesis to TAVI procedures-analysis of 50,846 aortic valve cases based on a Polish National Cardiac Surgery Database. Journal of thoracic disease, 11(6), 2340–2349. https://doi.org/10.21037/jtd.2019.06.04
[5] Prosthetic Heart Valve Market – Global Industry Trends and Forecast to 2027 | Data Bridge Market Research. (2021). Retrieved 2 April 2021, from https://www.databridgemarketresearch.com/reports/global-prosthetic-heart-valve-market
[6] Aortic Valve Replacement Devices Market: Industry Report, 2018-2025. (n.d.). Retrieved April 25, 2021, from https://www.grandviewresearch.com/industry-analysis/aortic-valve-replacement-devices-market
