
Designed for Cardiac Surgeons.
Meeting the needs of our customer while
Dedicated to Catching Calcium.
delivering innovative solutions aimed for our patients.
Meet the Team


BS in BME ’20, University of Rochester
MS in BME ‘22, University of Rochester
MD Candidate ‘26, Mount Sinai
Our Mission
Our mission is to significantly reduce post-surgical SAVR life-threatening complications caused by calcified debris – a major risk factor for embolic events like stroke. CalCatch envisions that the Calcium Catcher will transform the outcomes of surgical aortic valve replacement (SAVR) procedures, resulting in a considerable reduction in mortality and other high-risk events caused by calcified debris remaining in the heart due to inadequate techniques being used today.
Biodesign Statement
A device for cardiothoracic surgeons to capture embolic debris from falling into the left ventricle during surgical aortic valve replacements (SAVR) to reduce the number of particulates left behind and mitigate their associated risk of stroke.
Problem
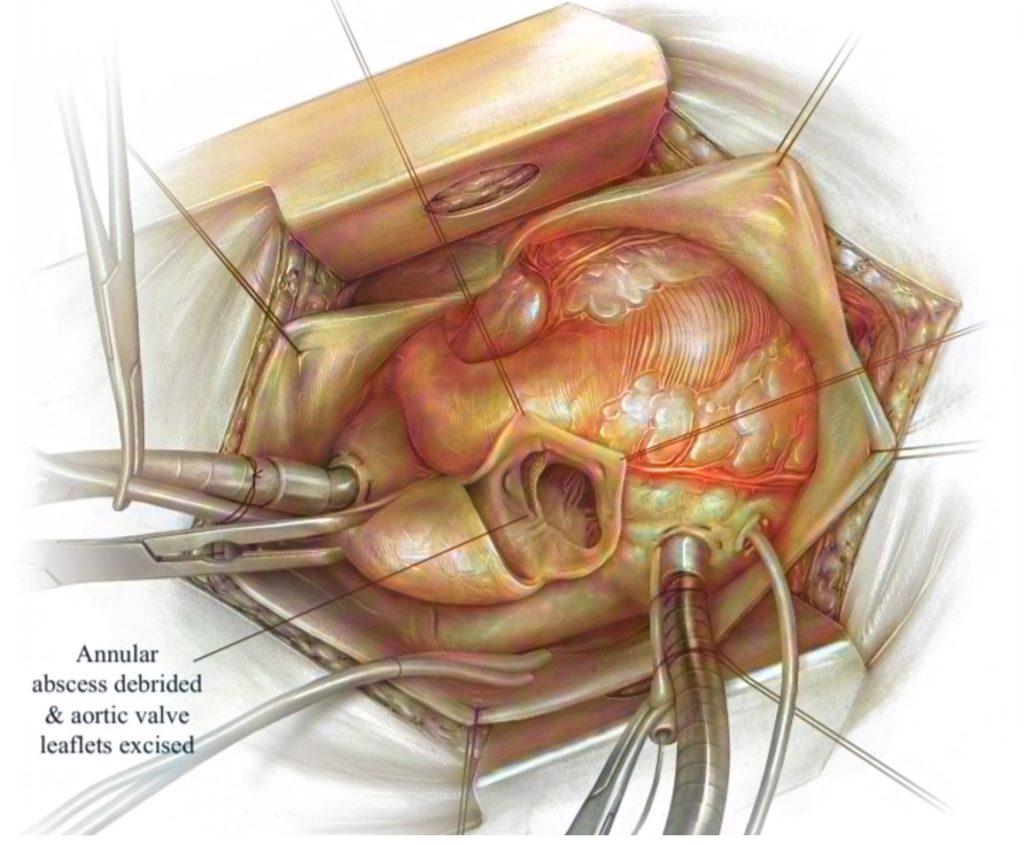
The leading cause of surgical aortic valve replacements (SAVR) is the build-up of calcium on the aortic valve, requiring the excision and debridement of the valvular leaflets before placing a new prosthetic valve. SAVR is expected to be a common procedure as the geriatric population expands and the number of Americans living with severe stenosis exceeds 1.5 million. CalCatch is introducing an embolic protection device for SAVR. During this procedure, calcified debris may fall into the left ventricle, as shown in Figure 1. Hence, when blood flow is restored, the embolic debris can enter the systemic circulation and cause embolic events such as stroke or cardiac arrest. As a result of the indirect causal relationship between dislodged debris and stroke rates, our team has developed a device currently not available in the medical device market, which addresses this unmet clinical need. This has been validated by our Key Opinion Leader for SAVR, Dr. Peter Knight, Chief of Cardiothoracic Surgery at the University of Rochester Medical Center (URMC). Our product was also endorsed by numerous URMC residents, including Dr. Clauden Louis and Dr. Nicholas Searcy, as well as the URMC Director of Value Analysis and Chief of Quality.
Current Solutions and their drawbacks
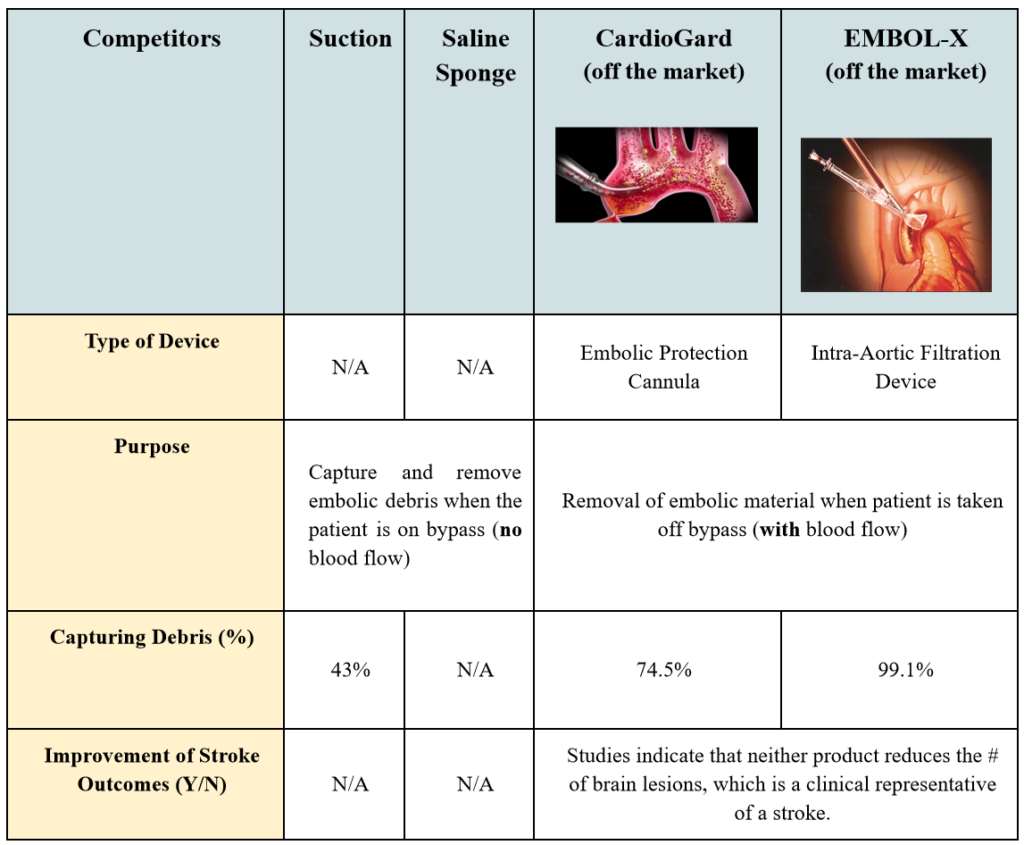
The Calcium Catcher does not have competing, commercially available technologies that capture calcified debris in SAVR procedures. Surgeons currently address this need with two approaches. The first is repeated use of suction while the other technique is the off-brand use of a surgical sponge doused in saline. The sponge gets packed into the left ventricle with no particular form or structure. Essentially, the goal for both of these techniques is to capture and remove most of the calcified debris. This is a vital component of the surgery because once the patient is taken off of the cardiopulmonary bypass (CPB) machine, the restored blood flow can dislodge any remaining debris into the circulatory system, which results in a stroke. However, how effective are these surgical techniques for ensuring that calcified debris is removed?
When observing the procedure in the operating room, the sponge is taken out in a manner that lends itself to dislodging some debris back into the left ventricle if not, at least, the microscopic level ones. The exact number of incidences of calcium embolization caused by any open-heart surgery is unknown. Sometimes the translocation of emboli to places other than the heart, brain, and kidneys is asymptomatic, which makes it difficult to determine the source of a stroke after such surgeries. Nonetheless, multiple attendings and fellows stated that a surgeon would always prefer to use a way to reduce chances for peri-operative stroke, which is strongly associated with calcium. Therefore, this is an unmet clinical need that still requires a device that would effectively catch calcified, stroke-inducing debris.
Our Product:
Calcium catcher
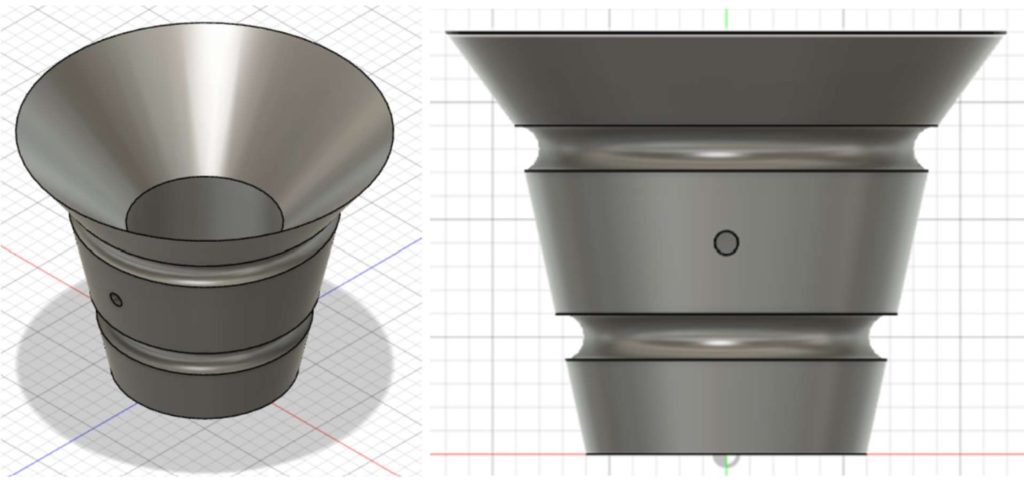
With the Calcium Catcher, cardiothoracic surgeons will now enhance patient-centered care with a novel device designed to mitigate the risk of stroke. The Calcium Catcher is a solution for cardiothoracic surgeons to limit the number of embolic calcium particles remaining behind in the heart following SAVR. Given its robust cylindrical design, the Calcium Catcher helps capture any calcium that falls from the aortic valve with its tapered top and optimized fifty-micron mesh (shown in Figures 2 and 3). The device’s balloon inflation mechanism offers surgeons a secure placement after deployment and better stability throughout usage.

Testing Plan
Test #1: Does the device stay in place?
Objective:
Determine how much CC of air (volume) is needed for the balloon to remain inflated and stay in place while supporting a weight of 55g.
Specification Tested:
The device shall maintain its position after deployment once inflated with less than 20 CCs of air.
Variable:
Record the amount of air needed to keep the device in place that can hold the weight of a 30mL cylinder (55g) for 10 seconds at least.
Results:
The final device needs at least 11 CCs with a 95% confidence interval of air to inflate the balloon such that the device can stay in place while supporting 55g in the air for at least 10s. The appropriate range is within 11-12 CC (rounded to the nearest whole number), which influences the choice of syringe that will come with the device.
Figure 3. Image of Set-Up for Test 1
Test #2: What percent of the calcium is caught in the device?
Objective:
Determine how much calcium can be caught by the device to suggest its effectiveness through the benchtop model indicated in Figure 4.
Specification Tested:
The device can catch at least 90% of calcium debris (size ranging upwards of 0.77 mm).
Variable:
Record the amount of calcium that was not captured in the device (fell into the box or on the rims of the benchtop model) to calculate the percentage of calcium captured by the device.
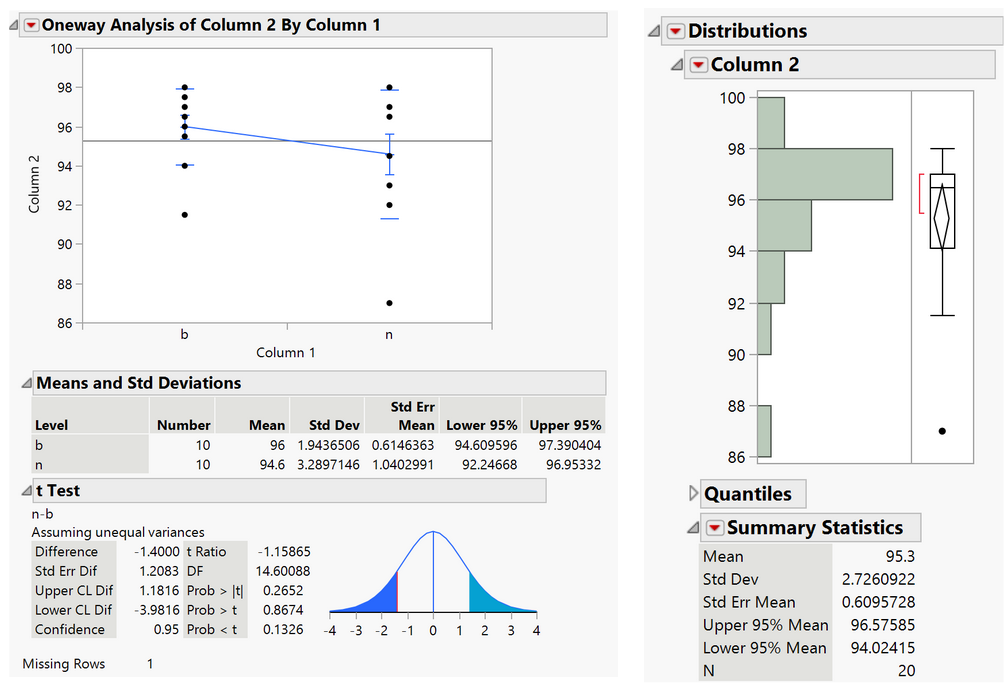
Results:
The range of the percent of calcium caught by the device is 94-97% with a confidence level of 95%, so this has exceeded the minimum specification value of 90%.
Test #3: Usability testing with Key Opinion Leader, Dr. Knight
Important Takeaways from User Testing Observations:
1. Dr. Knight would quickly push air into the device, which made it clear that there was no particular tactile feedback that helped the surgeon gauge how well the device is in place. However, he mentioned that the current prototype has thicker tubing, which made it difficult for him to visualize.
- Figures 5B and 5C demonstrate how he made a bigger incision to see the placement of the device and he stated that “the device fills the ventricle well.”
2. He suggested making it easy to image the device to confirm if the device properly fits below the annulus.
- We can incorporate barium coating such that the device appears white on x-ray film.
3. He was concerned about calcium that may fall when deflating the device before retrieval, so retrieving the device as it’s inflated while suctioning the crevice would be a good approach to mitigate this potential problem.
“An intruiging design and a positive start”
Dr. Peter Knight
Key Device attributes

REgulatory Strategy
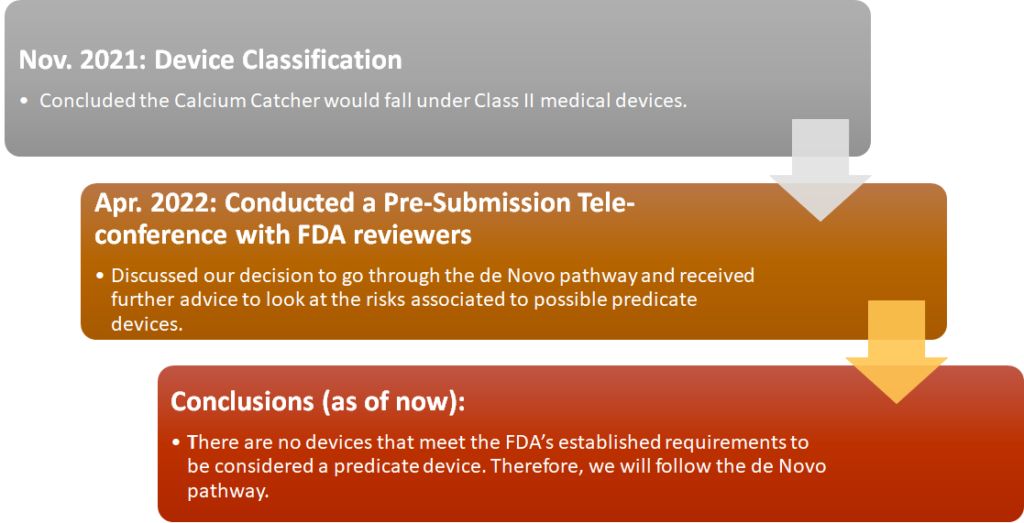
Business Model
Target Market
GOal: To Fill a Gap in the Market of embolic protection devices for SAVR procedures
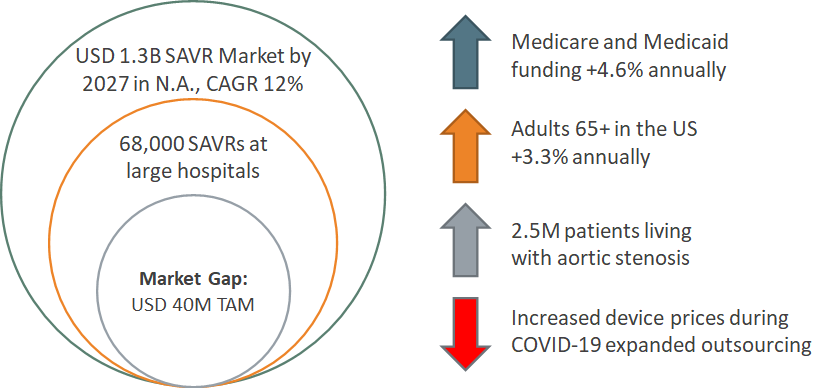
Competitive Advantage
This novel product does not have any commercially available technologies that have the same functionality of collecting and trapping calcified debris during open and minimally invasive AVR procedures. Although there is no competing technology, there is an emerging procedure competitor – transcatheter aortic valve replacement (TAVR/TAVI). TAVR is another clinical option that is gradually being adopted by many surgeons when treating patients with symptomatic, severe aortic valve stenosis. It has grown in popularity because it eliminates the need for general anesthesia, extensive surgical trauma, and a cardio-pulmonary bypass (CPB) machine. However, TAVI is a costly procedure upfront. Plus, recently published papers discussing beneficial post-TAVI effects may not be significantly different from the results from SAVR.
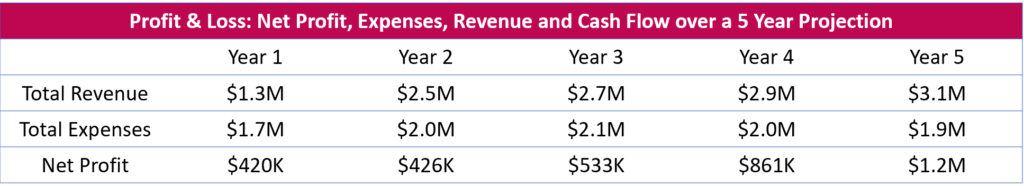
5 Year Financial Projection
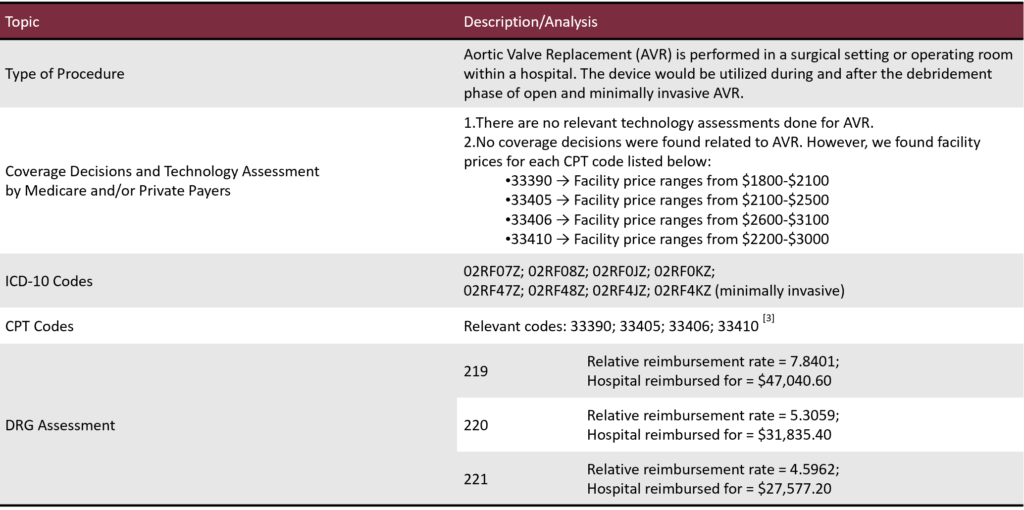
Reimbursement Pathway
FUTURE DIRECTIONS
Looking forward to optimizing the device design based on the tests discussed earlier and for manufacturing the final prototype, we plan to replace the needle with thin tubing that can be connected to the syringe and pressure sensor (similar to indeflators that are found in catheterization labs). Although we initially took the pressure sensor out of the design due to its bulkiness, we are planning to re-introduce it again based on VoC and reviewer feedback. In addition, we would also add barium to at least the rims of the inner funnel/cylindrical structure to allow surgeons to image the device during the procedure to confirm its placement of the device. Lastly, in regards to manufacturing this device in the future, we believe injection molding is a more feasible option than 3D printing the Calcium Catcher due to expenses. However, there will have to be strong communication and partnership between the manufacturer in charge of the inner structure and the balloon aspect of the device.
Acknowledgments
- Greg Gdowski (Executive Director of CMTI)
- Marty Gira (Senior Research Engineer
- Amy Lerner (Professor of Biomedical Engineering)
- Dr. Peter Knight (Chief of Cardiothoracic Surgery, Key Opinion Leader)
- Dr. Jonathan Stone (Neurosurgeon, Clinical Director)
- Mary Maida (Board of Directors at Daystar Kids, Business Coach)
- Mahllet Beyene (CMTI Graduate Program Coordinator)
Thanks to all our peers, collaborators, and clinicians for the support!





