Team


Ejiroghene’s LinkedIn 
Katarina’s LinkedIn
Biodesign Statement
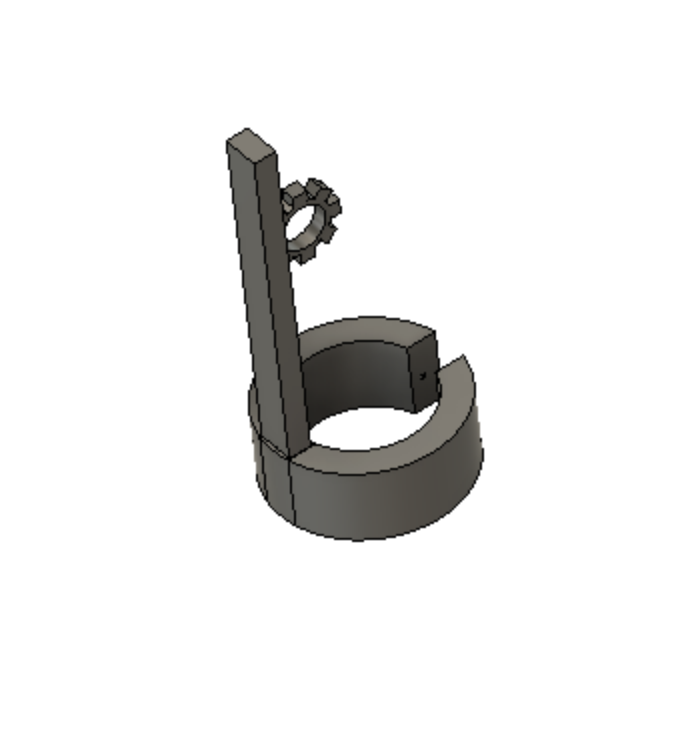
A device for cardiothoracic surgeons to use to retract the leaflets of the mitral valve to increase visualization of and access to the sub–valvular structures during mitral valve repair surgery.
Introduction
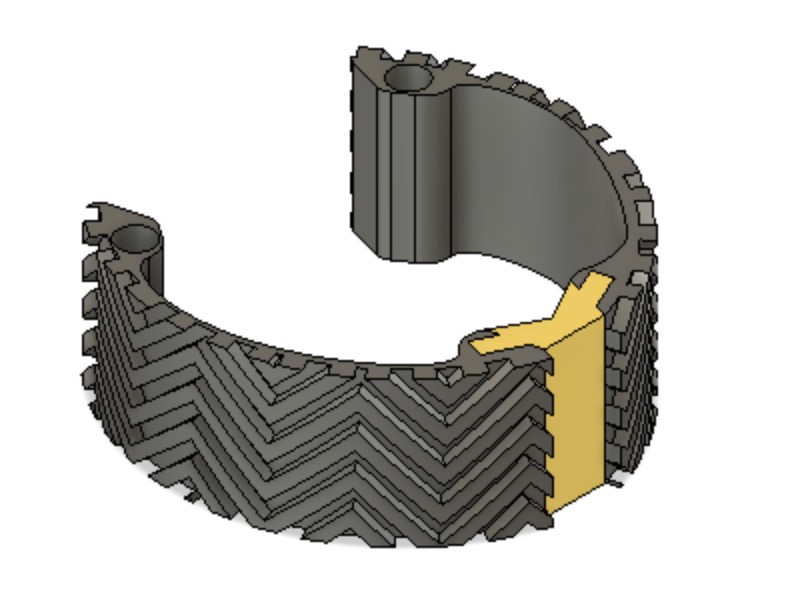
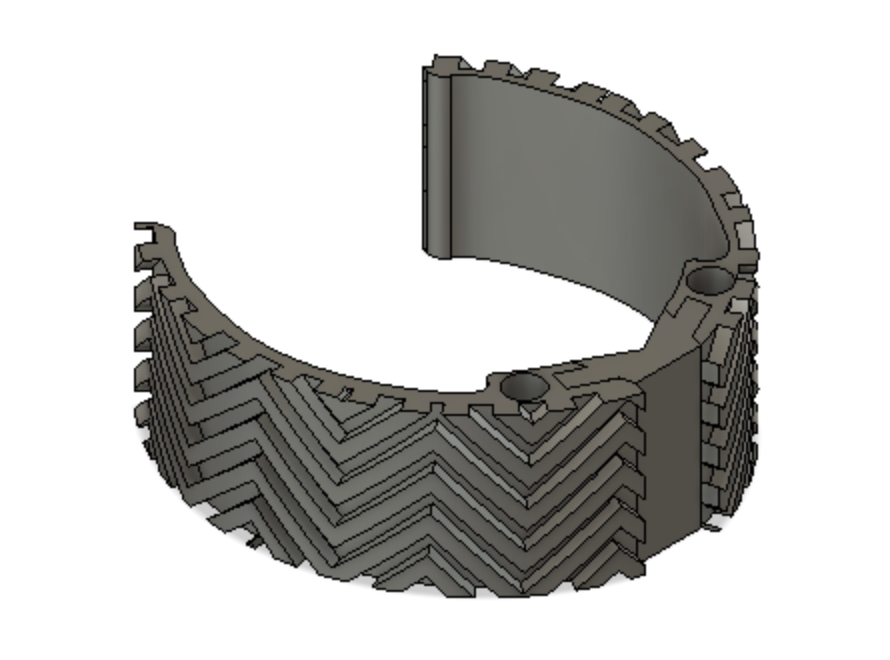
The Mitral Valve Papillary Ring is a one-of-a-kind mitral valve retraction device that aids in visualization of and access to the sub-valvular structures during mitral valve repair surgery. The device allows for hands-free exposure of the surgical site without the added risk of valve leaflet damage that is present with current methods of exposure. We are committed to providing cardiothoracic surgeons with the best methods for exposure to ensure an efficient surgical process.

Problem
The most critical aspect of a successful procedure during mitral valve repair surgery is adequate surgical site visualization. Proper visualization allows surgeons to recreate the patient’s native anatomy and improve hemodynamic function for better surgical outcomes. The primary method of obtaining visualization is suturing of the mitral valve leaflets. The secondary solution is the use of a sterile ruler. A few competitive devices on the market are also designed for mitral valve retraction and provide an advantage over traditional methods by reducing surgical time. However, the devices are significantly more expensive than rulers or sutures. The images below show each of the current methods of visualization and their primary shortcomings.

Our Device
Prototyping History
Click through the slide deck to see our iterative design process
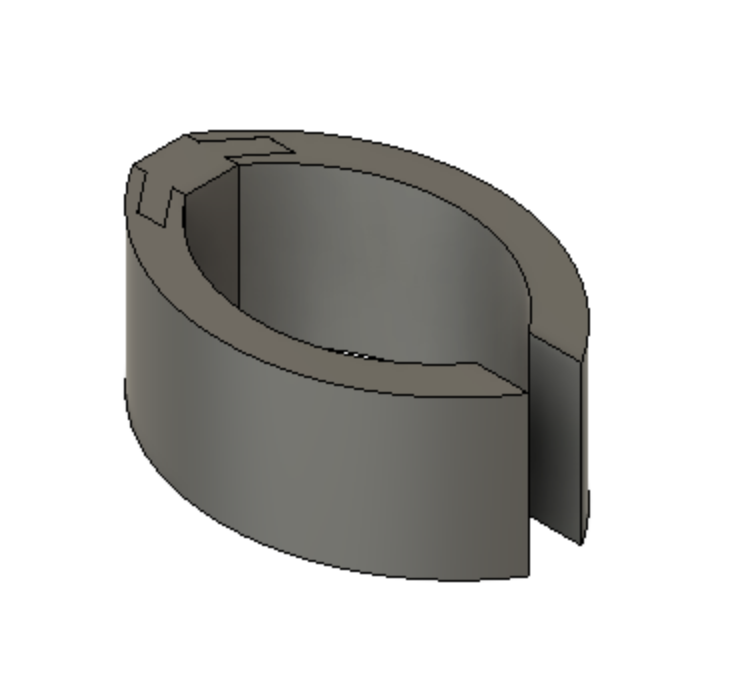
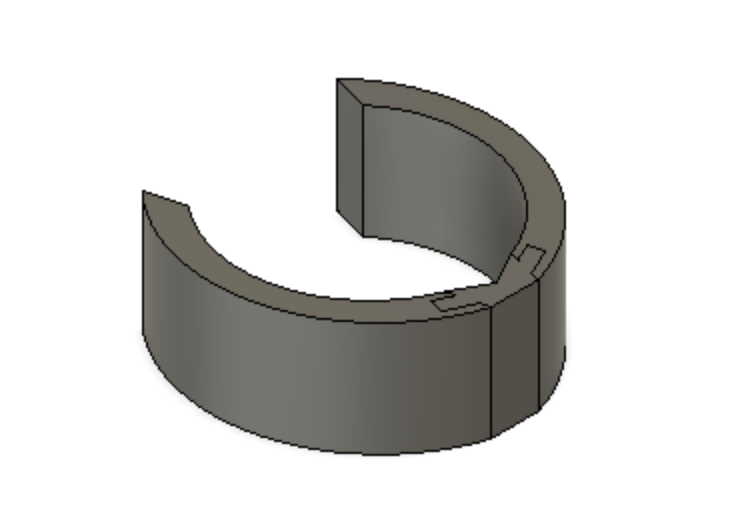
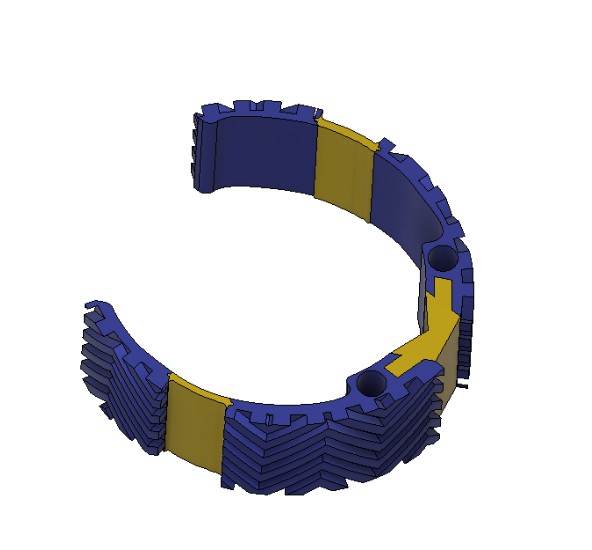
Device Features
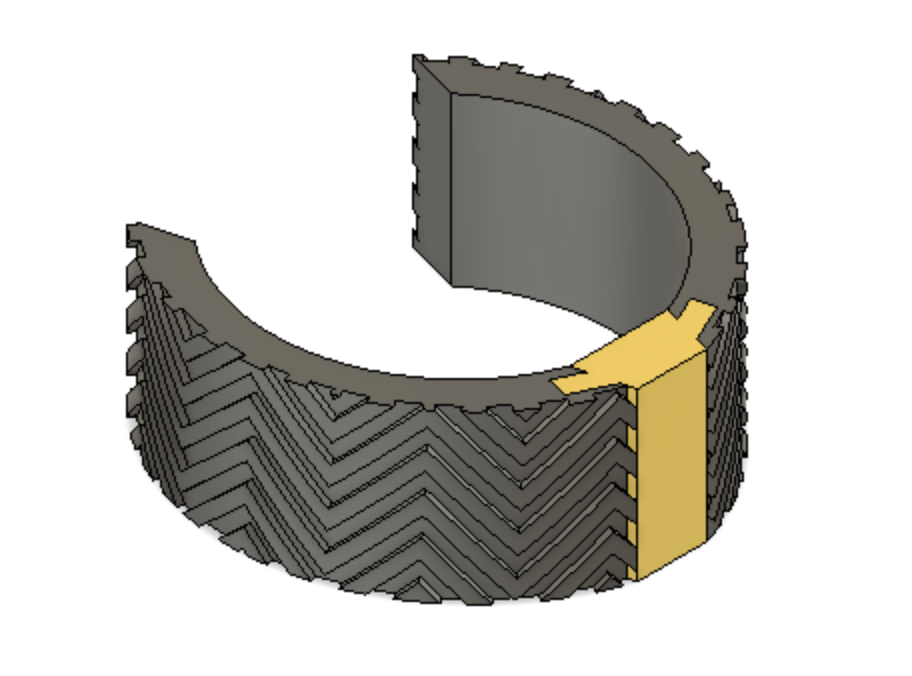
Optimally designed outer surface to promote stability and friction in the mitral valve
Deployment holes on the MVP Ring allow for efficient and atraumatic placement of the retractor into the mitral valve
Flexible, intercalated hinge
Dual material design
Mechanism for minimizing device upon deployment
Tailor-made for you!
We appreciate that our customers’ patients are unique. That’s why we design our retractors to suit your needs.
Below is an animation depicting the process of inserting the MVP Ring into the valve. The heart is shown cut open so that the viewer may easily see the anatomy surrounding the mitral valve. This view is not representative of a minimally invasive mitral valve repair surgery. The view during the surgery would be shown on a camera that would be inserted through the left atrium of the heart.
Voice of the Customer Feedback
Visualization really is the most important aspect of mitral valve surgery because it allows you to get close to accurately recreating the patient’s native anatomy.
Personal Interview with Dr. Daniel Ziazadeh (Cardiothoracic Surgery Resident, University of Rochester Medical Center)

Maintaining good stability in the valve is extremely important. When a device slips out of place in the valve, we have to take it out of the patient, roll it up and place it back in.
Usability Testing Session with Dr. Peter Knight (Chief of Cardiac Surgery at the University of Rochester Medical Center)

Better Than The Rest!

Heuristics Analysis
The team conducted a human factors and heuristics review of the Mitral Valve Papillary Ring in accordance with guidelines stipulated by IEC 62366: Medical Devices- Usability Engineering and ANSI/AAMI HE-75: Design of Medical Devices. Definitions for the various heuristics categories were obtained from the paper, “Using Usability Heuristics to evaluate patient safety of medical devices,” by Jiajie Zhang et. al (2003).
| Places of Occurrence | Usability Problem Description | Heuristics Violated | Mean Severity Rating | Design Modification | New Severity Rating |
| Assembly | The Scrub nurse adjusts the retractor to the wrong size. This could occur if the diameters that can be accommodated by our device are not well defined, and there is confusion about which size can best suit a patient. | Consistency, Match, Error, Document | 2.5 | We provide instructions for use as well as size charts with each pack of retractors | 1.5 |
| Mechanism of Adjustment | If adjustment is needed in this stage, it may be unclear what direction the surgeon is to manipulate the device in to make it smaller or larger in the annulus. | Consistency, Match, Memory, Feedback, Error, Language, Control, Document | 2.94 | We minimized the number of steps needed to adjust the retractor by 50% | 2.3 |
| Inadequate tactile feedback. This occurs because the ratio of the force applied to adjustments made is not well matched. Also, no stopping mechanism and a lack of audio or visual when they make each adjustment. | Consistency, Match, Error, Closure, Undo, Document | 2.93 | We tested the force exerted by our device and optimized the design to exert just as much force as devices that have been cleared to be on the market. | 1.5 | |
| Removal | The surgeon doesn’t decrease the diameter of the device prior to removal. | Consistency, Match, Memory, Error, Closure, Document | 3.75 | We intend to stress this as part of the device use and safety training we offer to surgeons. Also designed the device to suggest intuitive clamping of removal mechanism (but we understand that this is subjective) | 3 |
Testing Summary
The following tests were performed to optimize the device design and to prove the safety and effectiveness of our device. Sample size determination for testing was established in accordance with risk assessment guidelines stipulated in ISO 14971: 2019 (Application of Risk Management to Medical Devices)
Surface Adhesion Testing
With the goal of determining which features of the pattern are important for surface attachment, we performed a Design of Experiment (DOE) in order to optimize the patterned outer surface of our device. We performed a 4 factor, 2 level DOE. The factors were frequency of the peaks, density of the peaks, extrusion height of the pattern and wetness of the surface. This test was performed by dragging the patterned objects across a surface which was comparably similar to the surface of the mitral valve, and measuring the force used to translate the patterned object using a force transducer. A weighted cylinder was placed on the patterned object being measured to ensure that the force transducer would be able to record the value being measured. A free body diagram of the experimental setup is shown in the Figure below.

Factors Varied:
- Density
- Frequency of Peaks
- Extrusion Height
- Surface Wetness

Through analysis in JMP, we found that frequency was a statistically significant factor.

Force Testing
With the goal of determining whether our device exerts as much force on the valve leaflets as the state of the art design, we conducted force tests on the ruler and the MVP Ring. The ruler was tested in its rolled state to simulate the forces that it would exert on the valve leaflet tissue (Figure X). The MVP Ring was tested by fixing one arm and pushing the other arm from the open to closed position. Force was recorded using a Vernier device. Using statistical analysis the force from the ruler was compared to the force from the retractor. Using a two-tailed t-test it was found that the difference between the averages of the recorded forces was not statistically significant.


After comparing the force the MVP Ring exerts on the mitral valve leaflets with the force exerted by a current method of exposure, using a two-tailed t-test, we found that the difference between the forces was not statistically significant. A graphical representation of the results is shown in the Figure below.

Usability Testing
Preparation, Deployment, and Removal Time
During the process of obtaining visualization of the sub-valvular structures of the mitral valve, a patient is on cardiopulmonary bypass. Ideally, the amount of time that the patient must remain on bypass will be minimized during a surgery. In the usability test, we recorded the time that it takes a physician to prepare, deploy and remove our device. We used the ruler as the “gold standard” of comparison. Figure x shows our device after deployment into the mitral valve of a porcine heart. Figure y shows the average time for preparation, deployment and removal of our device compared to the ruler.



Customer Usability
A usability study was completed with Dr. Peter Knight. To obtain feedback on our design we had Dr. Knight utilize the ruler for visualization in a porcine heart, followed by our device. From the usability study we gathered that the patterned surface is a desirable feature of our design and helps to mitigate the largest problem that occurs with the ruler which is slippage within the valve. We also received feedback that for purposes of insertion into the surgical workspace our device arms should overlap to reduce the diameter of the device in the closed position.
The Market
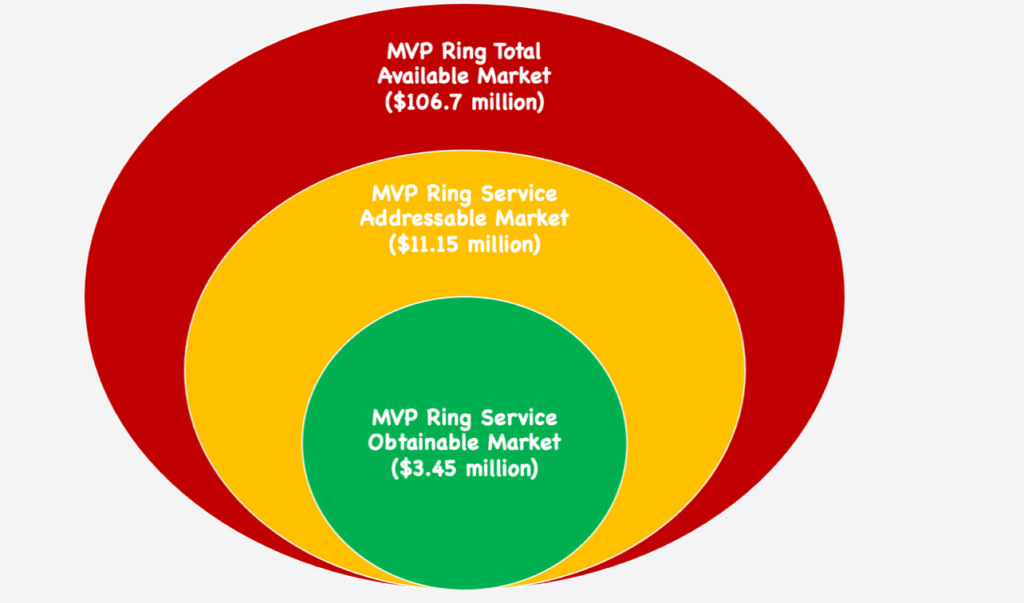
Significant Factors:
- About 44,600 Mitral Valve Repair Surgeries are performed in the U. S. each year
- Approximately 3-5% of the American population are affected by mitral valve prolapse
- Approximately 2% of the world population is affected by mitral valve prolapse
- The cardiovascular surgical instruments industry is estimated to be worth $1.41 billion, and it is growing with an increased prevalence of cardiovascular disease
*Our assumption for the Service Obtainable Market is that we are able to reach only 30% of surgeons performing this procedure in the United States
Key Takeaway: Our device has the potential to thrive in international markets based on market research and Voice of the Customer feedback received from internationally-based surgeons!
Regulatory Strategy
Considering other competitive devices in this space utilizing comparable technology, the team has decided to pursue FDA Clearance of the MVP Ring as a Class 1 device.
The product code for the MVP Ring is DWS which is a general classification for Class 1 Cardiovascular Surgical Instruments that are 510(k) exempt.
The MVP ring would still be subject to appropriate design controls and regulatory controls stipulated by the FDA.
The safety and efficacy of the MVP Ring have been evaluated in accordance with recommended FDA guidance documents, as well as internationally recognized standards including ISO 14971, ISO 13485, ISO 10993 and IEC 62366.
Future Work
- Material optimization
- Conclude the process of securing device patents
- Further biocompatibility testing
- Further animal testing is required
- Cadaver testing is also required to simulate near-surgical conditions
- Register team (company) with the FDA and begin the official path to market
Acknowledgements
- Dr. Greg Gdowski, CMTI Executive Director
- Martin Gira, CMTI Senior Research Engineer
- Dr. Amy Lerner, CMTI Academic Director
- Dr. Peter Knight, URMC Cardiothoracic Surgery
- Dr. Jonathan Stone, URMC Neurological Surgery
- Dr. Rajiv Devanagondi, URMC Pediatric Cardiology
- Dr. Maher Abadeer, URMC Pediatric Cardiology
- Dr. Martha Gdowski, URMC Department of Neuroscience