Team and Specialty
The General Surgery and Plastics Team is comprised of Anil Adharapurapu, Shruti Aryal, Phuc Do, and Jake Gilman. While most of the team rotated at the University of Rochester Medical Center under Dr. Galka and Dr. Schoeniger, Shruti completed her rotations at the Civil Hospital of Kathmandu under Dr. Vikal Shakya.
Medical Condition
Aiyan Gil is a 52 year old patient living with Type II Diabetes and a BMI of 36. She has been having severe pains in her upper right quadrant. Diagnosis revealed that she has bile stones in her gall bladder as well as the biliary tree. Her doctor recommends laparoscopic surgery to remove the gallbladder as it is the gold standard treatment for this condition.
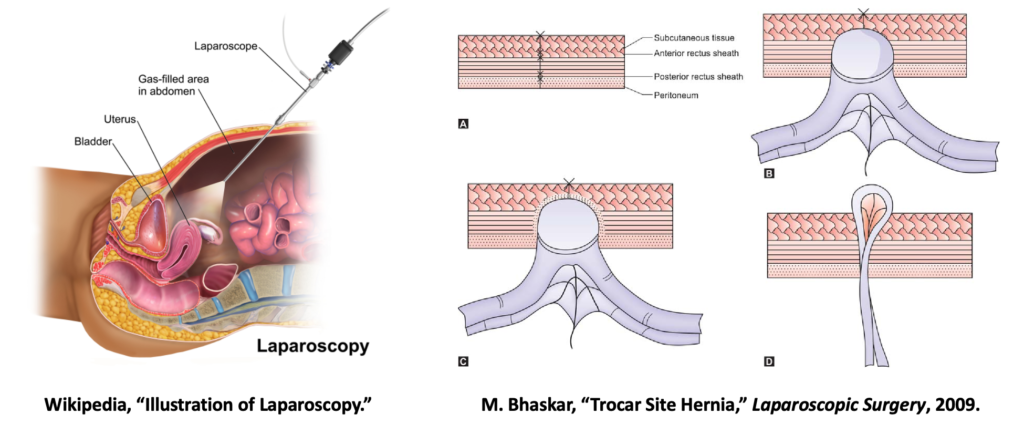
Laparoscopic procedures have gained popularity and are preferred over open procedures due to lesser degree of invasiveness, improved patient outcomes, and better cosmesis [1]. They can be performed for various conditions involving abdominopelvic organs such as gallbladder, liver, stomach, small intestine and urinary organs.
Surgical Difficulty/Problem
Our patient Aiyan has abdominal girth and heavy subcutaneous fat due to her size. This makes it more difficult for the surgeon to effectively suture up the trocar site wound at the end of the laparoscopic procedure. Twenty additional minutes had to be added to perform this last port closure step and even then the surgeon wasn’t entirely sure if the suture penetrated through the last layer of the fascia that needed to be closed. After the surgery, Aiyan was discharged as per normal protocol. But a week later, she returns back to her physician indicating pain at the port closure site. Further check up reveals that she has abdominal hernia and another surgical intervention would be required.
Herniation is one of the complications or risks associated with incisional wounds associated with laparoscopic procedure and has an incidence rate of up to 22% [2]. A safe, effective and less cumbersome intervention to seal the wound at port site, can prevent these complications from occurring.
Market Opportunity
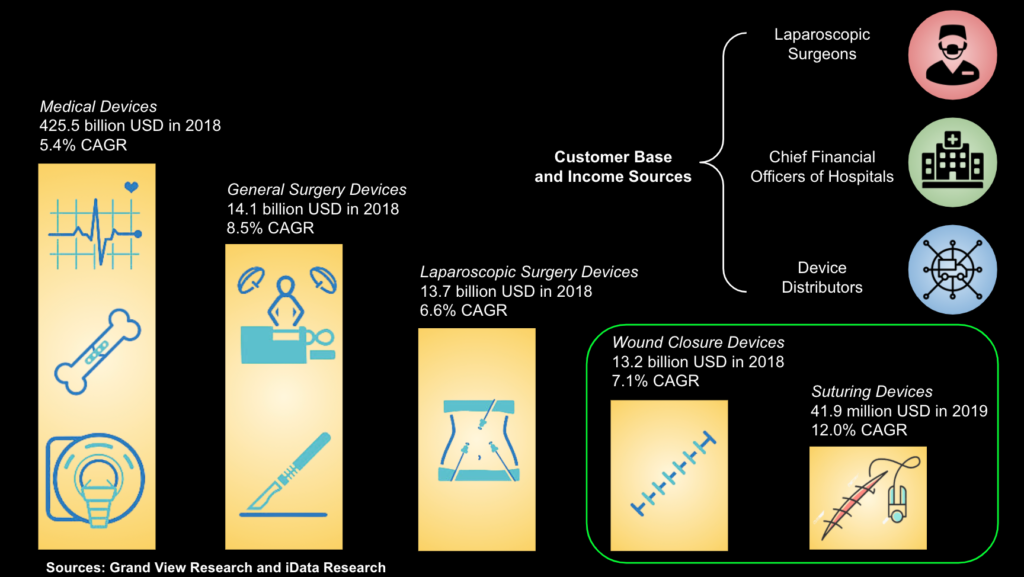
The mesh deployment device is designed to be used during laparoscopic surgeries. 15 million of these procedures are performed across the globe each year. Our project targets the wound closure device market, which was valued at $13.2 billion in 2018 with a compound annual growth rate (CAGR) of 7.1%. This market is segmented by mechanism of closure and includes adhesives, staples, and sutures. Surgical sutures and needles had the largest market share of wound closure devices at 41% in 2020, which translates to roughly $5.4 billion of the $13.2 billion total market value. A deeper dive into the market research showed us that most of this share went to the suture wires and hooks themselves, rather than mechanical devices that allow for surgeon-guided or automatic suturing of the wound. Since our company will compete with many suturing devices, we will primarily target the suture devices segment of the market, valued at $41.9 million in 2019 with a CAGR of 12.0% [3].
BioDesign Statement
To aid the wound healing process at trocar-inflicted port sites in patients undergoing laparoscopic procedures, as measured by decreased wound gap and reduced incidence rate of hernias.
Major Design Requirements
Over the course of the year, our team met with multiple surgeons to receive voice of the customer feedback on the requirements of our mesh deployment device. The following requirements were established by the team and considered during the design process:
(1) The device must be “quick-to-use”, allowing the surgeon to deploy the device, secure the mesh, and remove the device from the body in under 3 minutes. We utilized a toggle bolt mechanism to instantly deploy and retract the device and a suture guide for quick securement of the mesh.
(2) The device must contain an “easy-to use” mechanism of deployment and retraction. We decided on a push-pull plunger mechanism for smooth, controlled movements of the device and user-friendliness.
(3) The device must hold the mesh before it is secured to the peritoneal wall. We attached small hooks and velcro to the device to hold the mesh in the correct orientation when the device is deployed.
(4) The device must be waterproof and resistant to deformation. We made the device primarily out of plastic and waterproof materials so that it doesn’t degrade or deform under the external pressures of the abdominal wall.
Prototypes
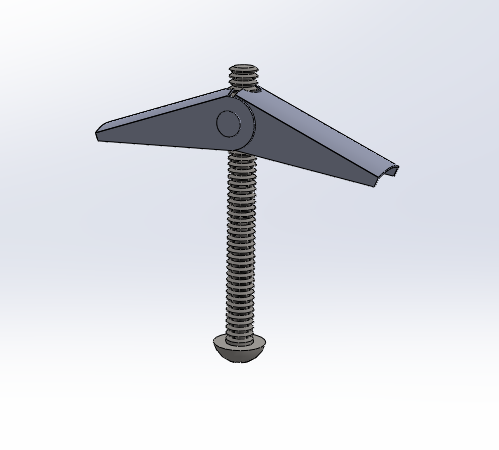
After several weeks of brainstorming and testing models in lab, our team was able to finalize a working prototype. Our device comprises of 2 main parts: the deployment device and the mesh.
The mesh is cut according to the size of the large trocar site in laparoscopic surgery: 20mm x 40mm. Suture is already embedded inside the mesh prior to packaging.

The deployment device‘s main body is a syringe-like tube. Its plunger is attached to a toggle bolt so that the toggle bolt can be pushed out of/pulled back into the tube. The wings of toggle bolt has hooks and velcro to keep the mesh in place during deployment. There’s an outer tube surrounding the device’s main body which houses the suture guide.

How It Works:
Device is inserted through trocar site, toggle bolt is deployed with the mesh, and mesh is oriented.

Suture is pulled out of body through suture guide, detaching mesh from deployment device.

Toggle bolt is retracted, device is removed, and mesh is secured by tying the suture.

Testing
The device is tested on the abdominal tissue plate from SynDaver to ensure the mesh can be deployed and suture correctly.

Since our actual device is smaller than our prototype (we made it big for demonstration purpose), simulation in SolidWork is performed to check the amount of force needed to operate our device.
Regulatory Strategy
Our device will be considered a Class II medical device by the Food and Drug Administration. This allows us to claim substantial equivalence to a pre-existing device and file a 510(k) Premarket Notification. The predicate device we chose is the AccuMesh Positioning System by Covidien LLC (K121139), which resides under product code ORQ. We would also file an investigational device exemption (IDE) to collect safety and efficacy data in future clinical trials.
Conclusions
After some time in the CMTI lab testing different design ideas, we were able to get a functional prototype that involved using a syringe, toggle bolt, 2x 3/16’’ nails, and hooks to hold the mesh before deployment. With the working prototype, the toggle bolt is able to deploy the mesh and then retract after the mesh detaches from the system. The mesh is then sutured using suture guides and an endoclose device. This method with the mesh deployment device would not only save time for the surgeon as well as allowing him/her to do efficient suturing, but also would prevent the patients from getting an incisional hernia during recovery.
Acknowledgments
We would like to thank Dr. Gdowski, Mr. Gira, Dr. Schoeniger, Dr. Galka, Dr. Stone, and Dr. Shakya for supporting us through the design process and providing crucial advice along the way.

