Team and Specialty

Aaron Craig 
Meg Luzenski 
Alex McMullen
Our team consists of Aaron Craig, Meg Luzenski, and Alex McMullen. We completed our clinical observations in the Orthopaedic Surgery department at the University of Rochester Medical Center (URMC).
Kaia Williams, a TEAM student, also joined the group to assist with our business plan. With Kaia’s help, our team made it to the semi-finals of the Mark Ain Business Plan competition.
Medical Condition
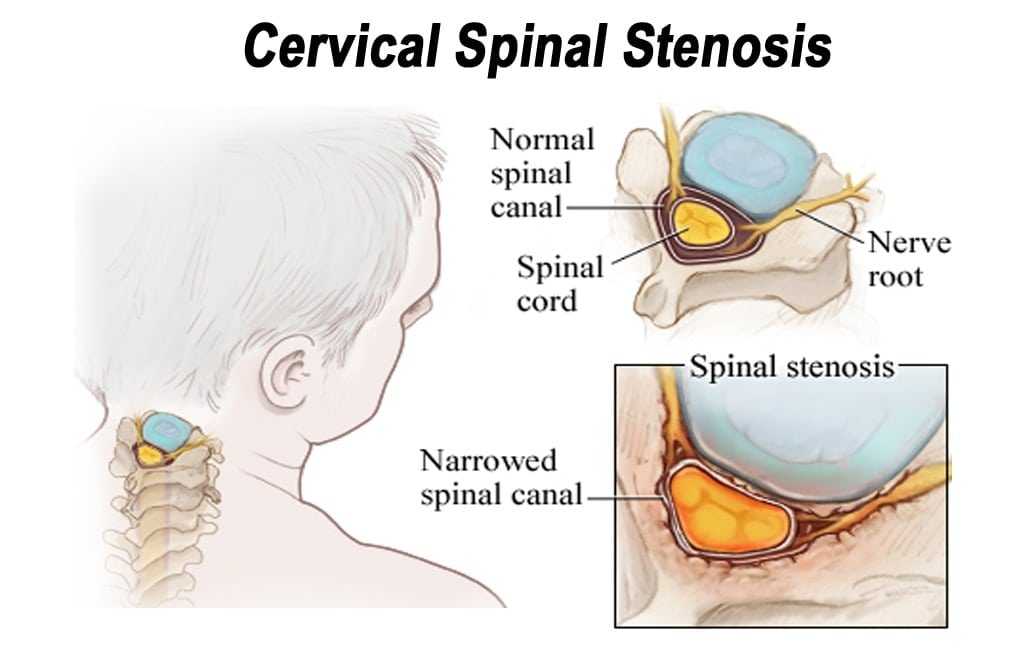
Source: https://discectomy.net/wp-content/uploads/2018/09/Cervical-spinal-stenosis.jpg
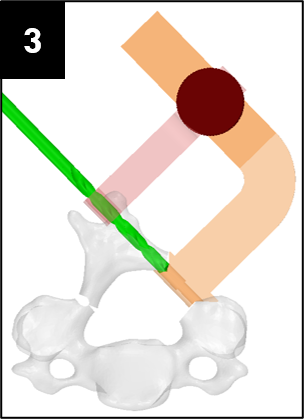
Cervical spinal stenosis is a painful, and potentially debilitating, narrowing of the spinal canal. In patients whose spinal stenosis is a result of cervical spondylitic myelopathy (CSM) or ossification of the posterior longitudinal ligament (OPLL) [1][2], an open-door laminoplasty procedure is a possible treatment option.
An open-door laminoplasty opens the laminae of cervical vertebrae to expand the spinal canal and decompress the spinal cord. When compared to Laminectomy or Anterior Cervical Discectomy and Fusion, laminoplasty has been shown to have lower complication rates and greater range of motion preservation [3][4][5].
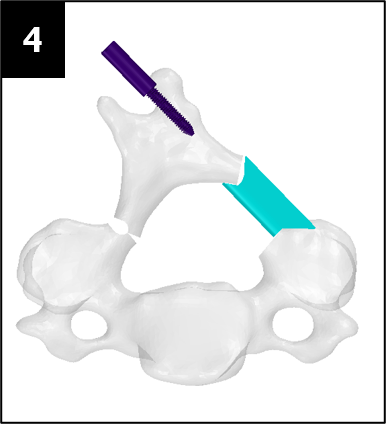
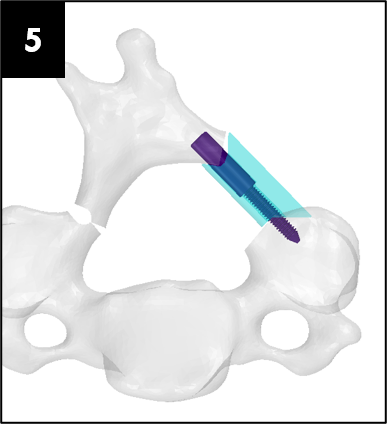
Source: adapted from the DePuy Synthes ARCH FIXATION SYSTEM surgical brochure
Surgical Difficulty/Problem
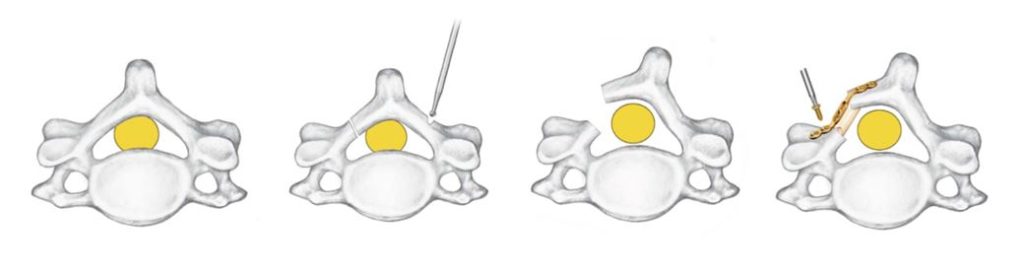
Currently available laminoplasty implant systems utilize 3 to 6 screws that are small and difficult to use. The size of these screws lends themselves to being dropped, posing a significant risk to sterility and patient safety.
These factors make an already challenging procedure more difficult, steering some surgeons away from the procedure altogether, despite the proven benefits to patients over laminectomies such as improved range of motion and reduced recovery time.
The current devices on the market all possess extremely similar design characteristics with little innovation over the past two decades. Some examples of current laminoplasty implants are shown below:






Market Opportunity
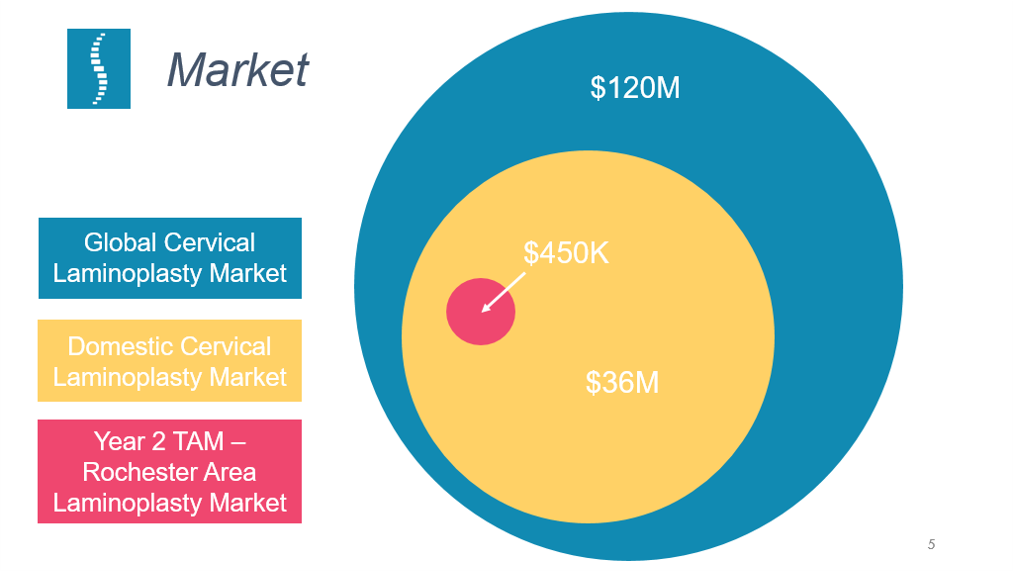
Source: from Portico Health business pitch slide deck [6]
Our market and sales strategy starts by engaging with surgeons that currently perform laminoplasties in the Rochester, NY region. We have estimated a total addressable market (TAM) of $450,000 in this region.
Our company believes that by creating a system that greatly simplifies the laminoplasty procedure we will not only be a part of the laminoplasty market but capture a portion of the laminectomy market as well, which could expand the available market by up to $105 million in the United States and $360 million globally.
BioDesign Statement
A way to eliminate the need for multiple bone screws during an open-door laminoplasty to make the fixation process more usable for spine surgeons.
Major Design Requirements
Our team has started documenting design requirements that will be used for eventual Design Verification & Validation (V&V) activities. Below are some of the critical requirements that we have identified:
Functional – Implant Fixation
The plate and fastener must remain fixated in the state they were in when they were implanted during the surgery.
Compatibility – Implant Material
The implant material must be nonreactive with the patient.
User Interface – Instrument Alignment
The drill guide and spacer must align concentrically.
Functional – Fastener Migration
Fastener remains in site of implantation after surgery and does not migrate.
Functional – Implant Mechanical Strength
Spacer and fastener can withstand forces experienced during daily activities of patients.
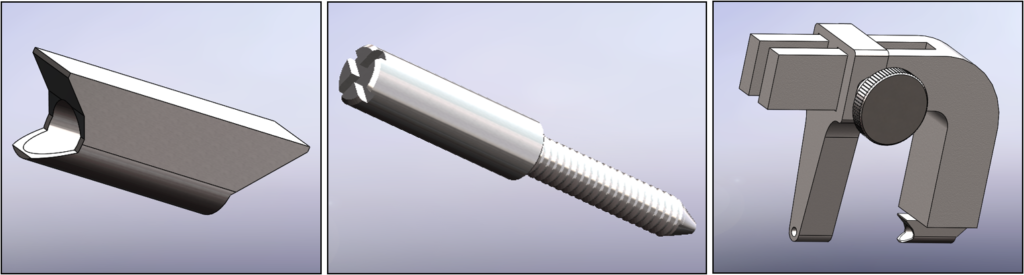
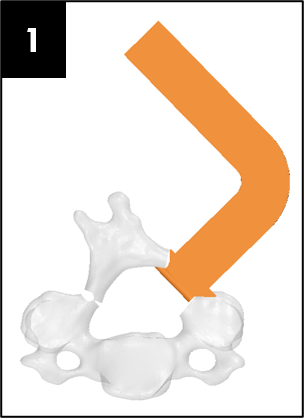
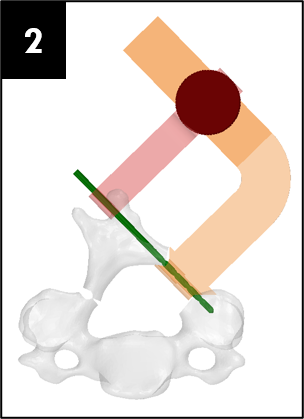
Prototypes
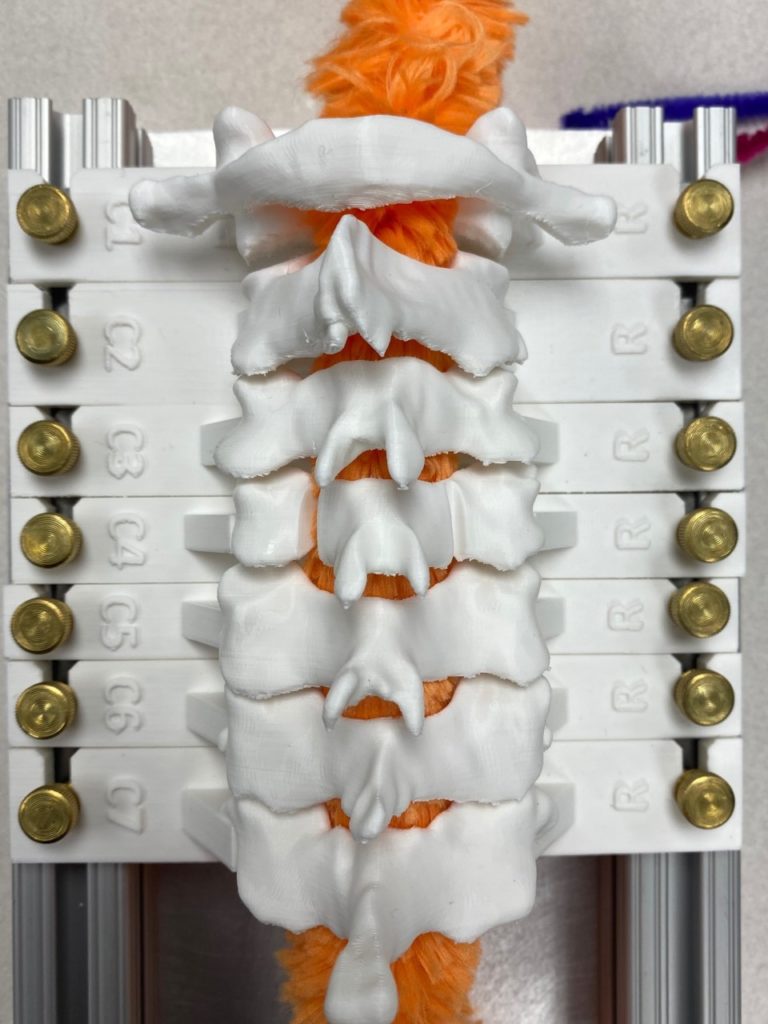
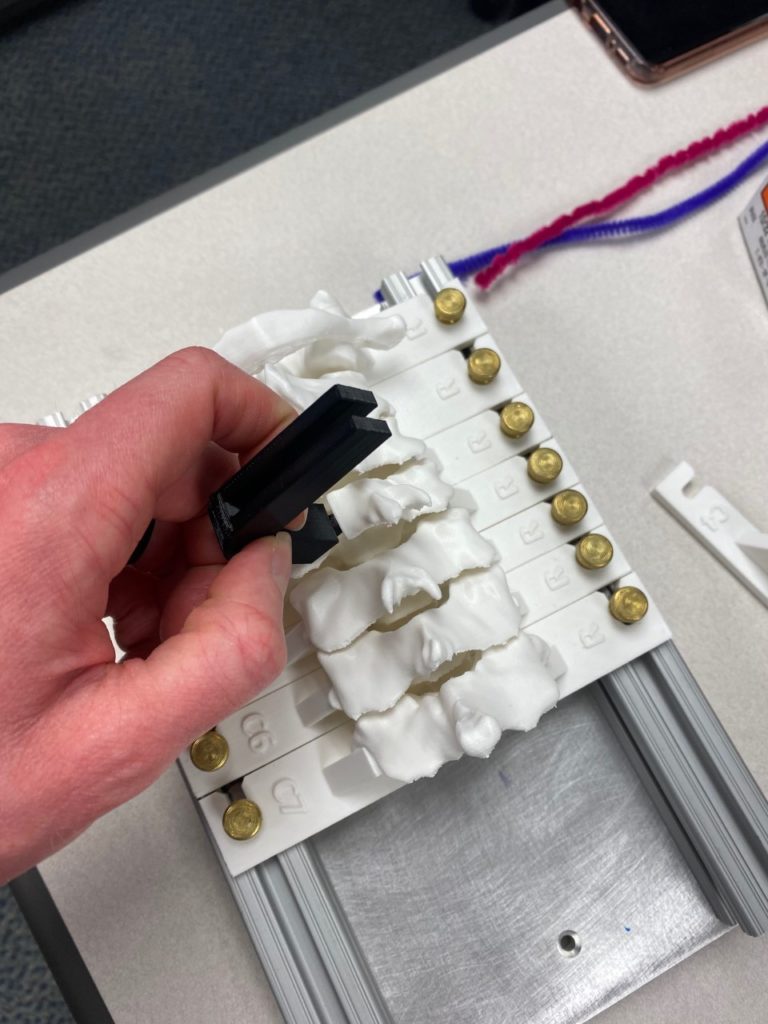
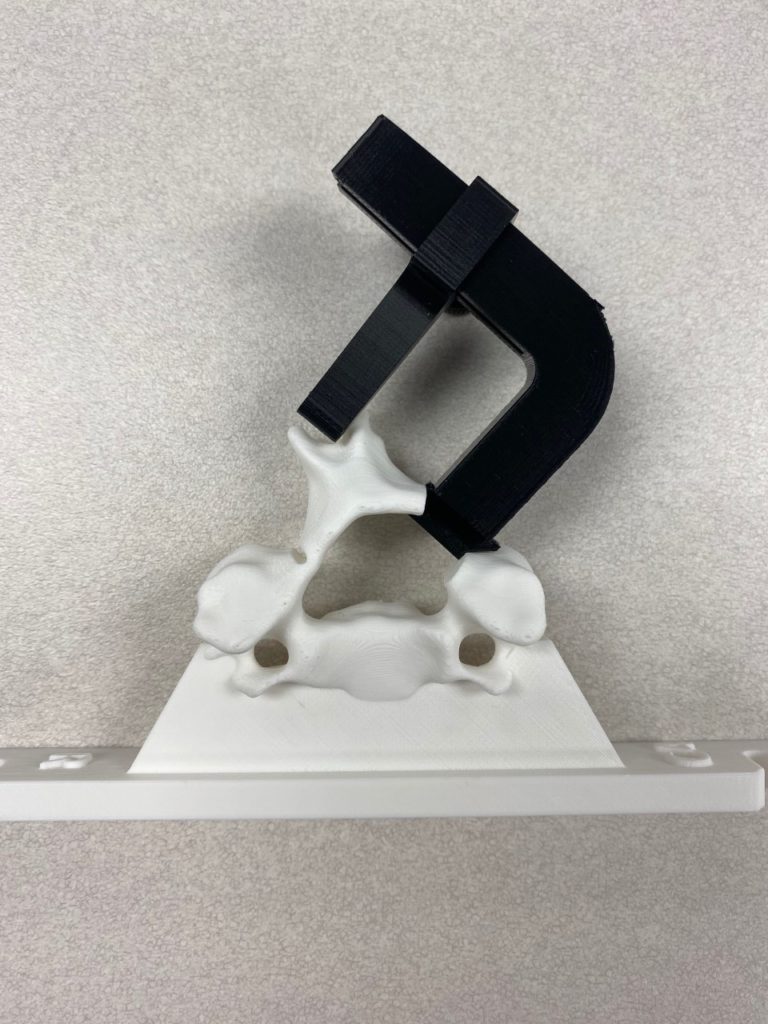
Our team was able to complete a first-iteration, proof-of-concept prototype to demonstrate our idea. This prototype includes working 3D printed models of the most common spacer implant size (10mm functional length) and a drill guide. A standard, off-the-shelf machine screw with the correct length and diameter is used for the physical prototype, but initial 3D models have been generated for the proposed fastener implant.
The images below illustrate how our system works:
Testing
Proof-of-concept testing has been completed for the first iteration prototype, using the 3D printed implant/instrument models and our custom-built laminoplasty test platform.







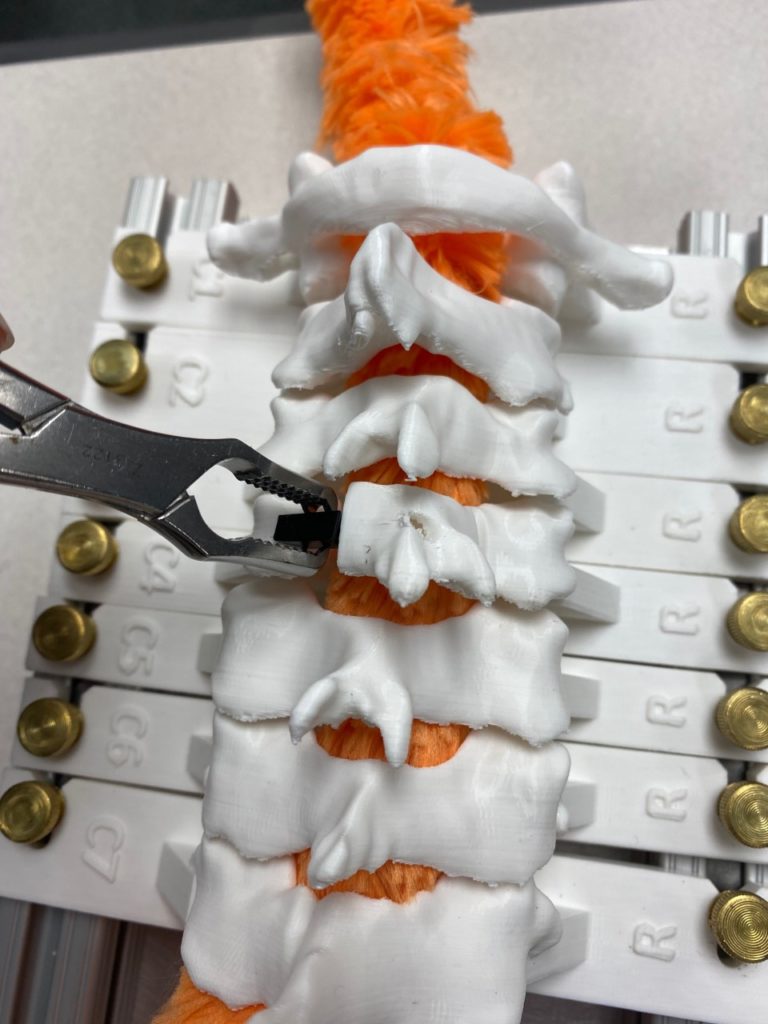
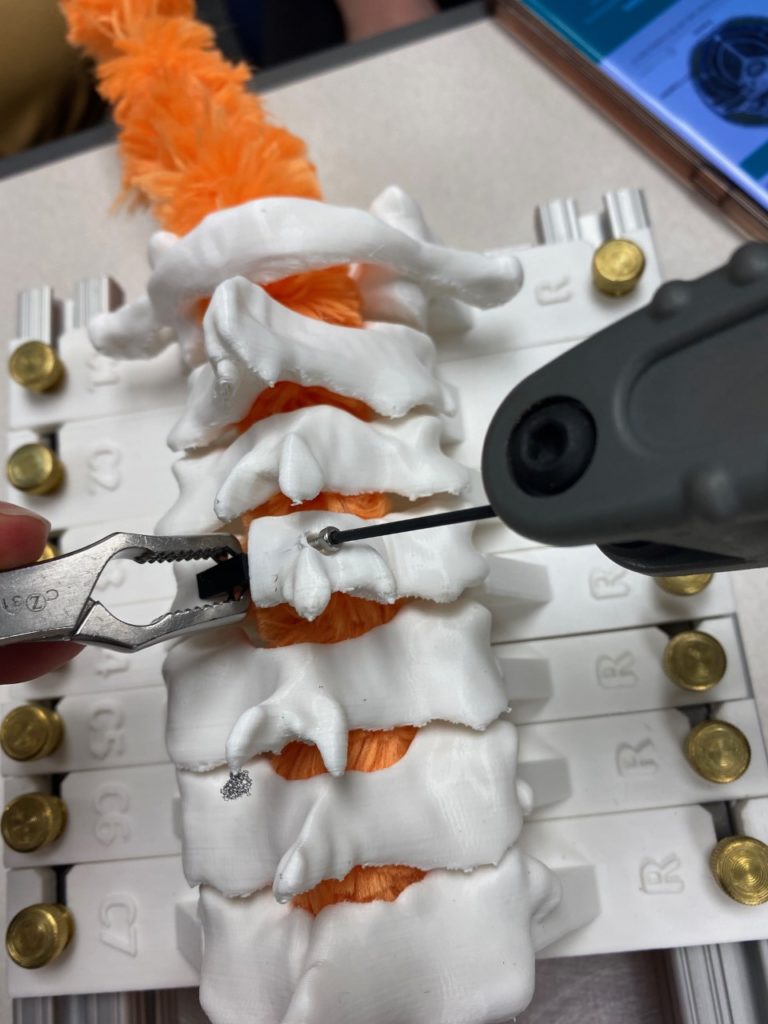



Future testing would include screw pull-out force, spacer compression strength, and quantification of decompression in the vertebral foramen.
Regulatory Strategy
Based on pre-existing regulatory framework for current laminoplasty implants, we have determined that the Portico Laminoplasty System falls into the following classification:
Product Code: NQW
Regulation Number: 888.3050 (Spinal Interlaminal Fixation Orthosis)
Class: II
Therefore, we propose that the regulatory pathway for this device will be through 510(k) clearance. This will require evidence of substantial equivalence to a predicate device, primarily through engineering benchtop testing. The device will have to follow general and special controls once cleared.
Conclusions
There is a clear need for innovation in the cervical laminoplasty space. The procedure offers a variety of benefits, yet some surgeons avoid it because of “finicky” and frustrating implants. Our team believes that we have developed a novel implant system that makes open-door laminoplasty more approachable. Further work needs to be completed to refine the design and conduct appropriate testing to ensure this device is safe and effective.
Acknowledgments
We would like to thank our fantastic team of mentors and clinical collaborators for their guidance and contributions to this project:
- Greg Gdowski, PhD
- Martin Gira
- Mary Maida, PhD
- Addisu Mesfin, MD
- Howard Silberstein, MD
- Shalin Shah, MD
- Jonathan Stone, MD
We would also like to thank the CMTI program and the University of Rochester Biomedical Engineering department for giving us this opportunity.
References
[1] Steinmetz, M., & Resnick, D. (2006). Cervical Laminoplasty. The Spine Journal, 6(6), S274–S281. https://doi.org/10.1016/j.spinee.2006.04.023
[2] Shimer, A., Lee, J., & Tannoury, C. (2007). Operative Techniques in Orthopaedics. Laminoplasty, 17(3), 169–173. https://doi.org/10.1053/j.oto.2007.04.003
[3] Adogwa, O., Huang, K., Hazzard, M., Chagoya, G., Owens, R., Cheng, J., Ugiliweneza, B., Boakye, M., & Lad, S. P. (2015). Outcomes after cervical laminectomy with instrumented fusion versus expansile laminoplasty: A propensity matched study of 3185 patients. Journal of Clinical Neuroscience, 22(3), 549–553. https://doi.org/10.1016/j.jocn.2014.10.001
[4] Wang, M., Yokoyama, T., Numasawa, T., Toh, S., McGrady, L., & Toth, J. (2006). P121. Biomechanical Evaluation of a Modified Cervical Laminoplasty. The Spine Journal, 6(5), 141S-142S. https://doi.org/10.1016/j.spinee.2006.06.330
[5] Hale, J. J., Gruson, K. I., & Spivak, J. M. (2006). Laminoplasty: a review of its role in compressive cervical myelopathy. The Spine Journal, 6(6), S289–S298. https://doi.org/10.1016/j.spinee.2005.12.032
[6] Williams, K. (2021, April). Portico Health Business Plan. Mark Ain Business Plan Competition. Rochester, New York.