Team and Specialty
Our team consists of Jared Ocasio, Nina Stash, and Charles Patterson. We founded the clinical need for which our project is based upon during our observations in the orthopaedic surgery department at the University of Rochester Medical Center.
Medical Condition
Spinal Stenosis is the pinching of the spinal cord and is caused by many factors. Pertinent to this device is stenosis caused by overgrowth of the bone surrounding the spinal cord, mainly the facet joints and lamina. To fix this cause of spinal stenosis, one procedural option is a laminectomy. This procedure involves the removal of the spinous process, lamina and part of the spinal facet. These components of the vertebrae, as well as causes of spinal stenosis are shown below in Figure 1. It is important to note that only spinal stenosis caused by bone spurs and overgrowth are fixed by a laminectomy.
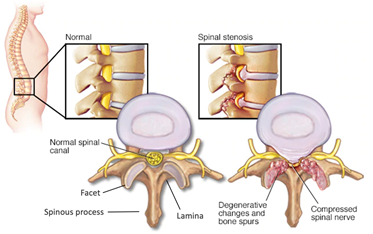
Figure 1. Labeled vertebrae and visual representation of spinal stenosis.
Surgical Difficulty/Problem
In laminectomy procedures, part of the spinal facet, a small bony joint connecting vertebrae, are removed to relieve pressure on the spinal cord. Currently, surgeons estimate the size of the facet by eye, a subjective and often inaccurate process. Removing too much bone can cause destabilization of the spine and require further surgery whereas removing too little can cause symptoms to persist. Figure 2 below demonstrates the facet that is currently only measured by eye.
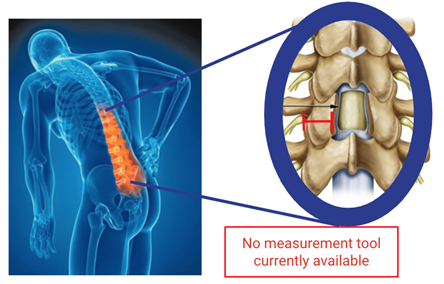
Figure 2. Highlighted in red is spinal facet width that our solution measures.
Solution
Our solution is a discrete measuring tool that can measure the initial width of the spinal facet and then be set by the user to some fraction of this distance to determine the goal for the final width of the facet. This tool can further be used to measure the width of the facet multiple times throughout the procedure of removing bone off the facet. This solution helps with multiple aspects. First, medical students, residents, fellows and any other student of this procedure could use this tool as they are learning to provide objective measurements of the facet and how much to remove. Some experienced surgeons can remember this by eyesight, but even for experienced surgeons, this tool provides objective measurements that can improve their consistency of medical care.
Market Opportunity
Lumbar spinal stenosis (LSS) affects more than 200,000 in the United States every year. Between 2002 and 2007 the incidence of spinal stenosis surgeries among Medicare beneficiaries was 135.5-137.5 per 100,000, with 500,000 laminectomies performed per year in the US. The mean cost for decompression with fusion is $80,888 and the total cost spent on hospital bills for LSS among Medicare beneficiaries was $1.65 billion. A market report from July of this year forecast the global spine implant market to reach $13.81 billion in 2023 at a compound annual growth rate of 5.31%. This market consists of revenue generated by the companies manufacturing spine implants used to correct deformities in the spine, stabilize and strengthen the spine, and facilitate fusion of bones.
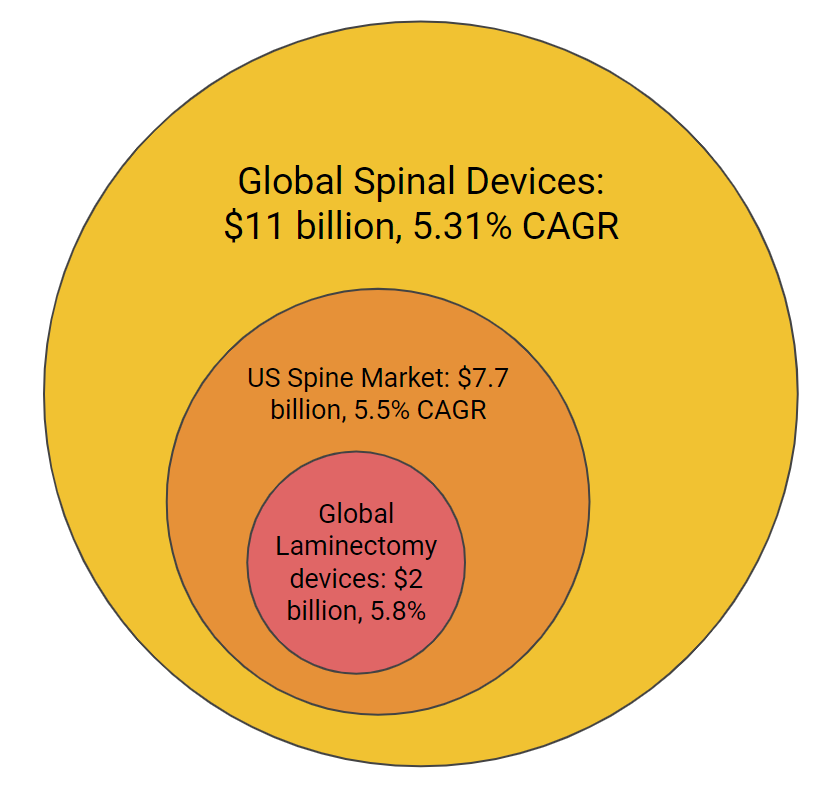
Figure 3. A breakdown of the market size for which our device fits into.
BioDesign Statement
A device for surgeons to quantify the width of a vertebral segment while removing components of a facet joint during laminectomies.
Major Design Requirements
In the table below we list out the major design requirements for our device. These requirements were born out of conversations with surgeons, out advisors, and standards for human factors.
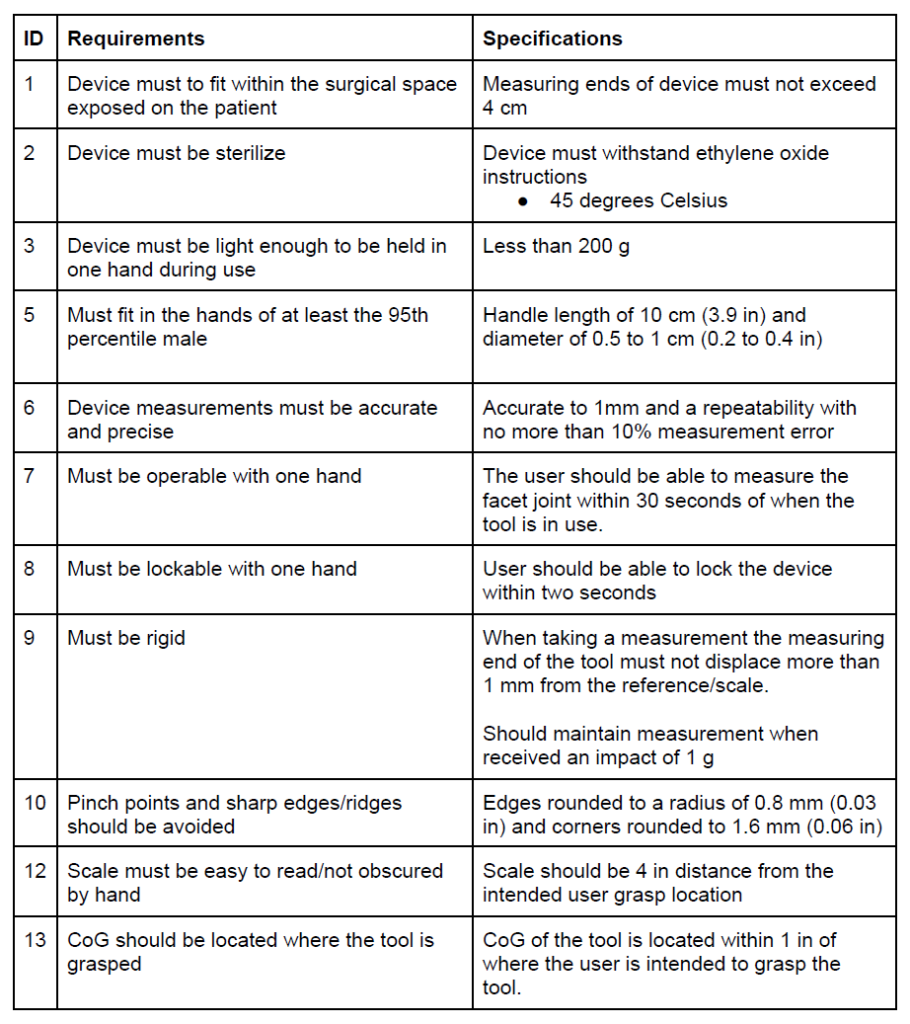
Table I. Design requirements and specifications
Prototype
Our design was inspired by the mechanism used on compasses and methods for holding and using chopsticks. These inspirations in conjunction with the previously identified design requirements led us to a novel design for a surgical caliper.
Our latest prototype enables the surgeon to obtain the width measurement with one hand, allowing them to have a free hand for holding back a retractor, using suction, etc. Additionally, we incorporated a locking mechanism so that the surgeon may take the measurement, lock the caliper, and remove the instrument from the surgical site in order to read the measurement.

Figure 4. CAD rendering of our latest prototype.
Testing
Multiple tests will be performed in the next two weeks on our device. Before delving into the tests, one must understand the overall process of using the device, shown below in Figure 5. It is important that this device accurately measures the facet width and adequately locks or holds in pace so that the surgeon can visualize this locking.
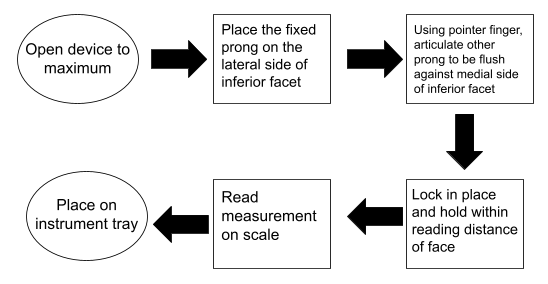
Figure 5. Flowchart for use.
Test 1 – Full Procedure:
The device shall be used in a setting simulating the actual use as shown in the flowchart above and will be completed on a spine model inside a model of the back. The test will be repeated with each user and the candidates will include surgeons and CMTI students. Each candidate will complete a survey on ease of use and the date from each measurement will be analyzed with gauge R&R.
Test 2 – Gauge Blocks:
Gauge blocks of 0.5cm, 1cm, 2cm, 4cm will be measured with the device by multiple candidates (surgeons and CMTI students), with multiple trials. This data will be analyzed with gauge R&R.
Test 3 – Drop Test:
The locked device will be dropped from 10cm, 30cm, 1m and 2m at different orientations of the device, with 50 trials each. The device will be both analyzed for wear and tear and its ability to lock in place.
Regulatory Strategy
Our device will be considered Class II by the FDA under the product code NQW, meaning we will need to submit a 510(k) Premarket Notification as well as an investigational device exemption (IDE) for clinical testing. The predicate device chosen for the 510(k) is Epic Anterior Thoracolumbar Plate System (K202309). which is also a Class II device.
Conclusions
The prototyping of this device is finalized and our main VOC, Dr. Addisu Mesfin, has loved the device. In the next two weeks leading up to the exit interview, our group will complete all testing and make any refinements to the design that arise from testing. The most finalized form will be presented in the exit interviews.
Acknowledgments
Our team would like to acknowledge Dr. Greg Gdowksi, Martin Gira, Dr. Jonathan Stone, Dr. Addisu Mesfin, Dr. Steven Feldon, Dr. Robert Molinari, and Dr. Shalin Shah for their continued support throughout this project.



