Final Device Results
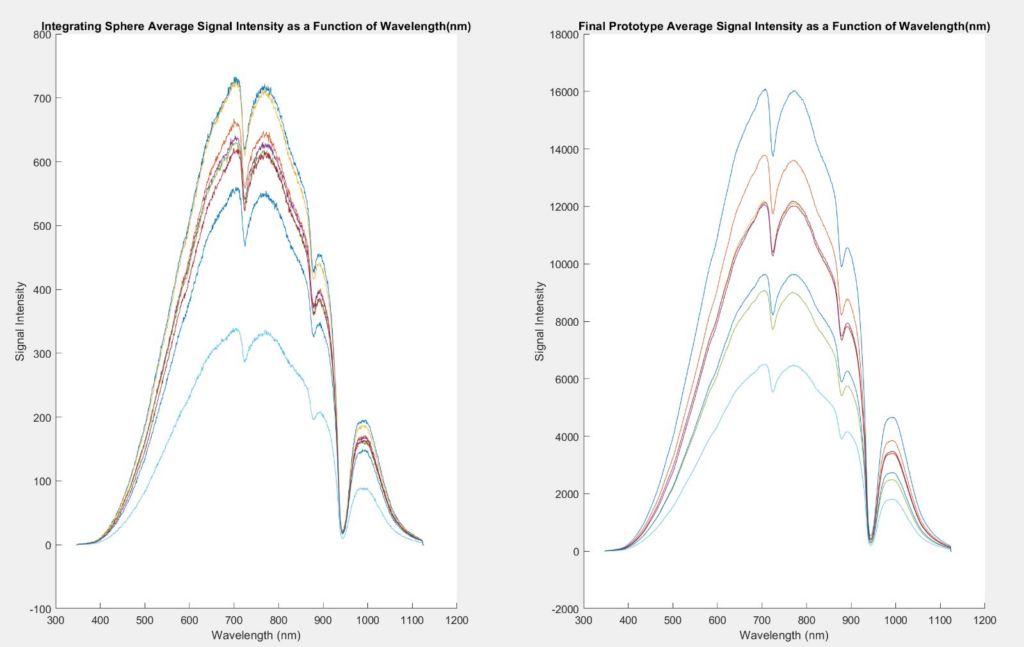
Stage 1 testing went as planned, and we were able to prove that our device had indeed produced more reliable, precise results than the integrating sphere measurements. Stage 2 testing showed us that each geometry had similar results in their standard deviation, so we chose a spherical cavity for our final device.
We also performed a brief test to optimize the height and width of the device and found that having at least 20 mm was enough to prevent the probe from exiting the device and to keep it perpendicular to the exit hole into the chamber.
At this point we felt comfortable in having our final device machined out of the heat resistant plastic, PEEK. The schematics for the two parts of the device are attached as follows:

Stage 3 testing is still underway, but so far, a few cycles have shown minimal differences in the surface of the Spectralon. We also confirmed that the inner chamber exceeds temperatures of 138°C using temperature sensitive test strips, which satisfied the sterilization criteria for autoclaves.

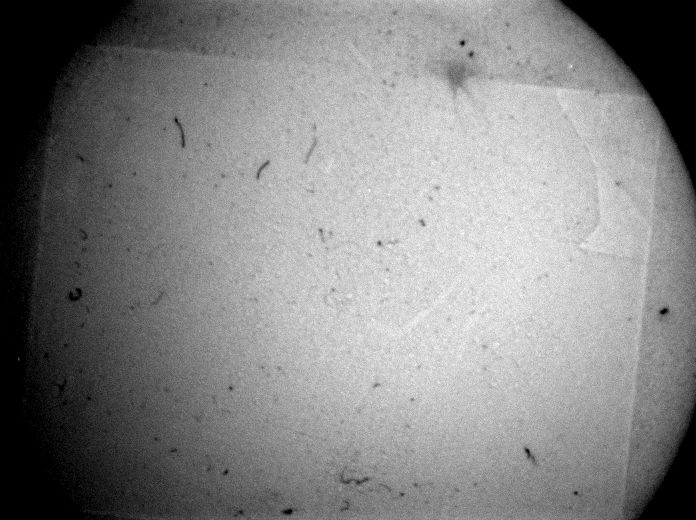
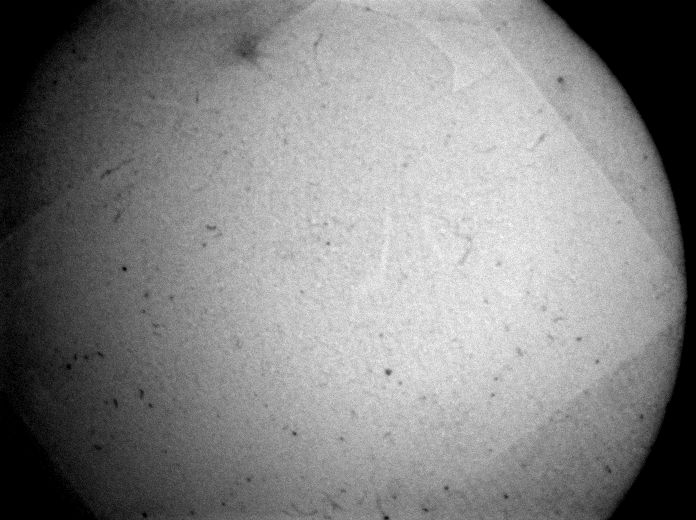
PEEK plastic can be autoclaved hundreds of times before showing significant wear, so the final device succeeds in what we needed it to do. Looking into the future, a precision system that does not rely on a preplaced o-ring is ideal. This can be far easier to achieve with a modified probe as well which our customer is interested in doing at some point.
