Problem Statement
Our goal is to design a tamper-evident device that incorporates an antiseptic component to protect the needleless connector of all vascular access lines, reducing the risks and associated costs of central line bloodstream infections (CLABSI) caused by intentional and unintentional tampering.
Risks Associated with Tampering
- About 28,000 patients die from CLABSI annually (Ahrq.gov, 2012)
- Excess costs associated with CLABSI is $70,696 per patient (Ahrq.gov, 2012)
- Annual total costs of care associated with CLABSI is $98-245 million (Ahrq.gov, 2012)
Customers for our Device
The main users would be the nurses and providers responsible for placing and removing this device and the patients on whom it would be placed.
The patient population that this device is designed for has broadened to include all types of vascular access, which include everything from PICC lines to regular peripheral IV lines. The device is intended to inhibit both intentional and unintentional tampering that could lead to harm of the patients.
Therefore, our second user population includes various groups of patients, especially those at high risks for developing CLABSI. For example, this includes patients with intravenous drug use history (past or present), patients who are mentally and cognitively impaired, confused patient with Alzheimer’s or dementia, such as children, patients with neurodegenerative diseases, patients with psychiatric disorders, patients with traumatic brain injuries (TBI), and pediatric patients.
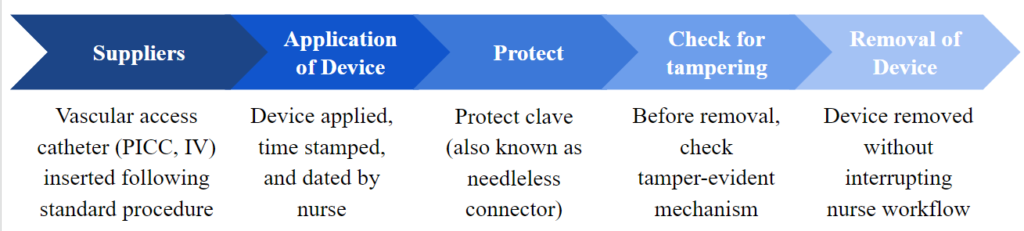
General Use Scenario
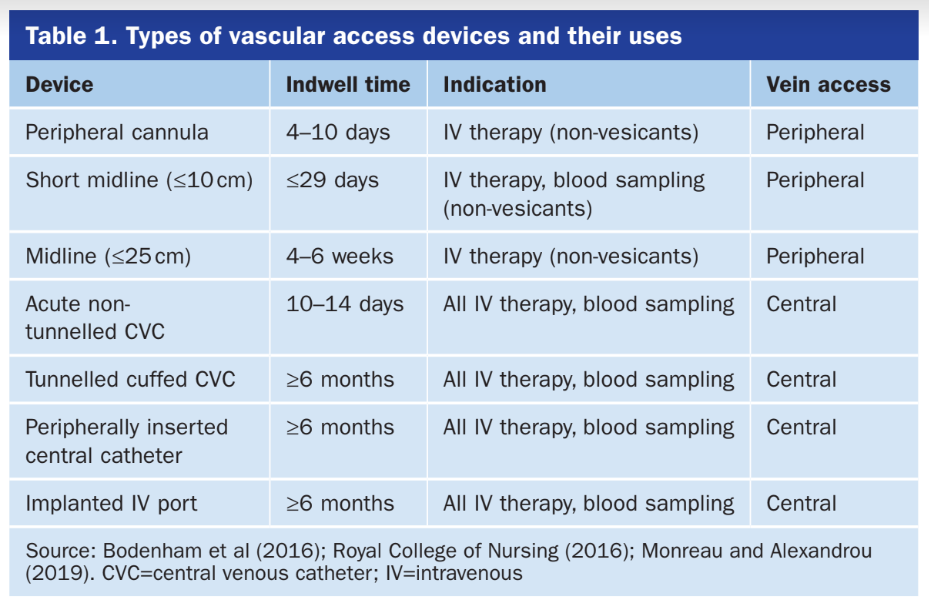
As we are making this device to be universal to all patients with vascular access lines, there are multiple types of lines that our device has to be compatible with. The list of these lines is in the table below (Barton, British Journal of Nursing, 2020). To note, the tunnelled cuffed CVC line is used for dialysis and the device would not be used on those lines as it is a protected line.
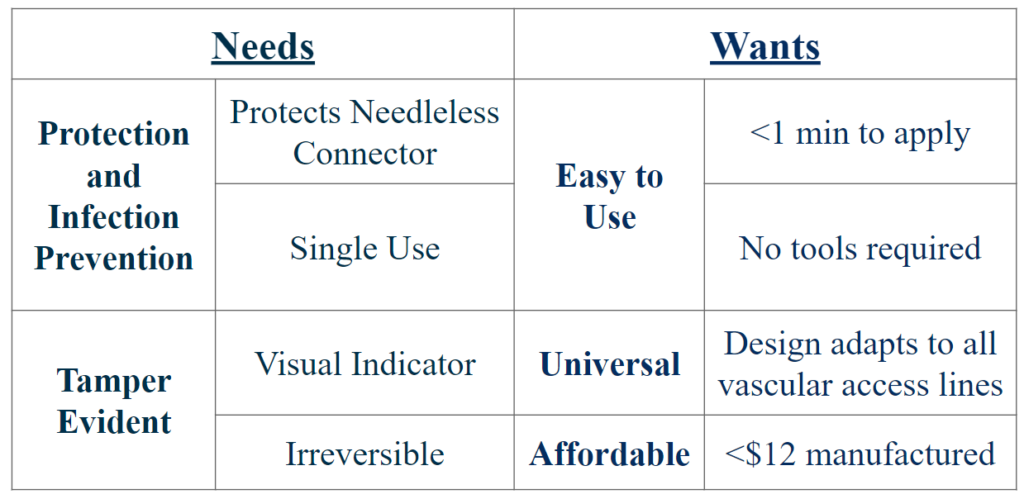
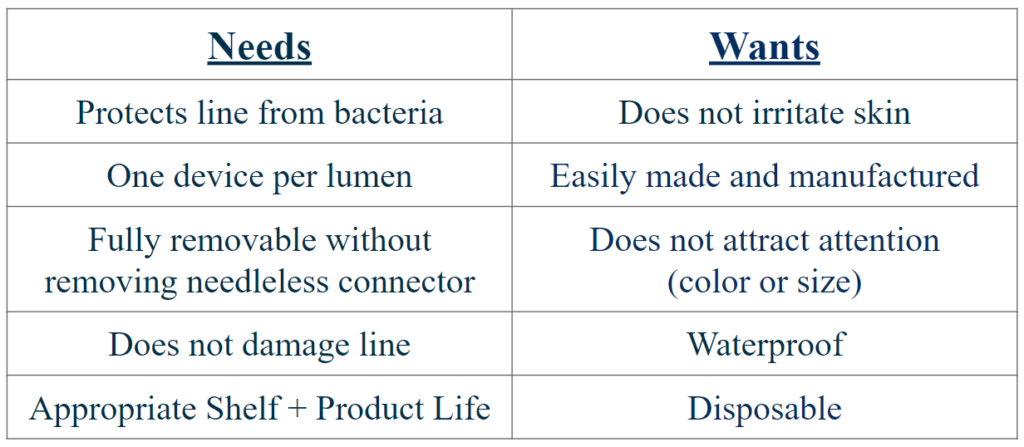
Needs
Prototypes


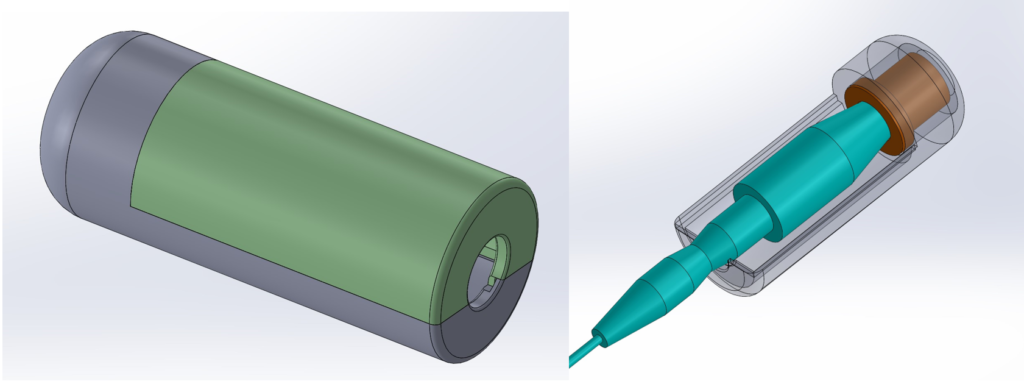
Testing
| Criteria | Req. | Test | Timeline |
|---|---|---|---|
| Tamper evident | Y/N | Sample testing if subjects can identify tampering | Spring 2022 |
| Nurse workflow | Y/N | Sample testing to measure average time to learn, apply and remove the device accurately | Spring 2022 |
| Universal Product | Y/N | Physical testing of dimensions over different types of vascular access lines | Spring 2022 |
| No tools required | Y/N | Sample testing of subjects can apply and remove the device without aid of external tools (like scissors) | Spring 2022 |
| Criteria | Req. | Test | Timeline |
|---|---|---|---|
| Infection prevention | Y/N | Material testing for disinfection+ Sample testing for save removal | Future |
| Protects Needleless connector | Y/N | Mechanical testing to measure force needed to break the device | Future |
Testing Results
We had the opportunity to test our device with URMC nurses from different departments. Here are some of the first impressions on our device:
- “Device is more discrete and less embarrassing”
- “Helps prevent infection by protecting the needleless connector”
- “Flimsy, but also bulky”
Besides gathering the first impressions and intuitive thoughts about our device, our team also tested how the device would impact the nurse workflow. This was done by measuring the time it took read the instructions for use (IFU) manual, correctly apply and remove the device from a PICC line. For this specific test, the average time it took to read instruction for use was 32 seconds (n =3). The average time it took to apply the device on a PICC line was 22.5 seconds with a success rate of 85.7% (n=7). The average removal time was 10 seconds with a success rate of 80% (n=5).
Lastly, our team tested the tamper-evidence property of our device. This was done by presenting 2 devices to the subjects. The subjects were tasked with identifying which of the two devices had been tampered with. Our team recorded the time it took the subjects to come up with an answer and whether the answer was correct. Our results showed that 80% (n =4) of the subjects were able to come up with answer under 5 seconds. The results also show that 100% (n=11) of our subjects could correctly determine whether or not our device had been tampered with.
Overall, our testing revealed some aspects of the device that needed to be changed to make our device easier to use. The size of our device was also re-evaluated after this testing and the feedback was incorporated in our final design.
Next Steps
Economic constraints
- The main competitor for our device is the PICC Guard. The price of their device is $12.64 (PICC Guard, 2022). Our goal is to be less expensive than the PICC Guard, but ideally our device would be sold for $5 or less.
- If the PICC Guard is put on a patient and changed 12 times a day (the maximum number of times a line would be changed in one day), it would cost around $1060 for a one week stay, which translates into $6370 if the device is used for 6 weeks (the longest time a PICC line would likely be in a patient).
- Using our device for one week would cost $420, and this would translate to $2520 if used for 6 weeks.
Manufacturing constraint
- There were limitations with 3D printing for our devices mainly because of the overall small size of the device as well as the need for flexibility for some of the components of the device.
- Injection molding was evaluated as the most efficient manufacturing method for mass production of our device because of its benefits. Those benefits include allowing scaling up of production, allowing manufacturing of many identical parts and the fact that the cost of productions gets lower as more parts are manufactured (1, 2, 3)
- Injection molding is however expensive mainly because of the high cost of machinery and molds. The process is also more applicable for mass productions hence cannot be used to manufacture just a few prototypes. (1, 2, 3)
1. Tremblay, 2012
2.Twi-global.com, 2022
3.Wisconic, 2022
Intellectual Property
- Prior Art competitors on the market include the PICC Guard (patent application with 20 claims) (7) and Neuma Clamp (patent application of 8 claims) (8)
- All claims are narrow and focus on the closure of the enclosure of the device and sliding mechanism
- This makes it easy for another company( us in this case) to design around the existing patents
- Our claims would also be narrow as well and it would be challenging to prevent others from building off it
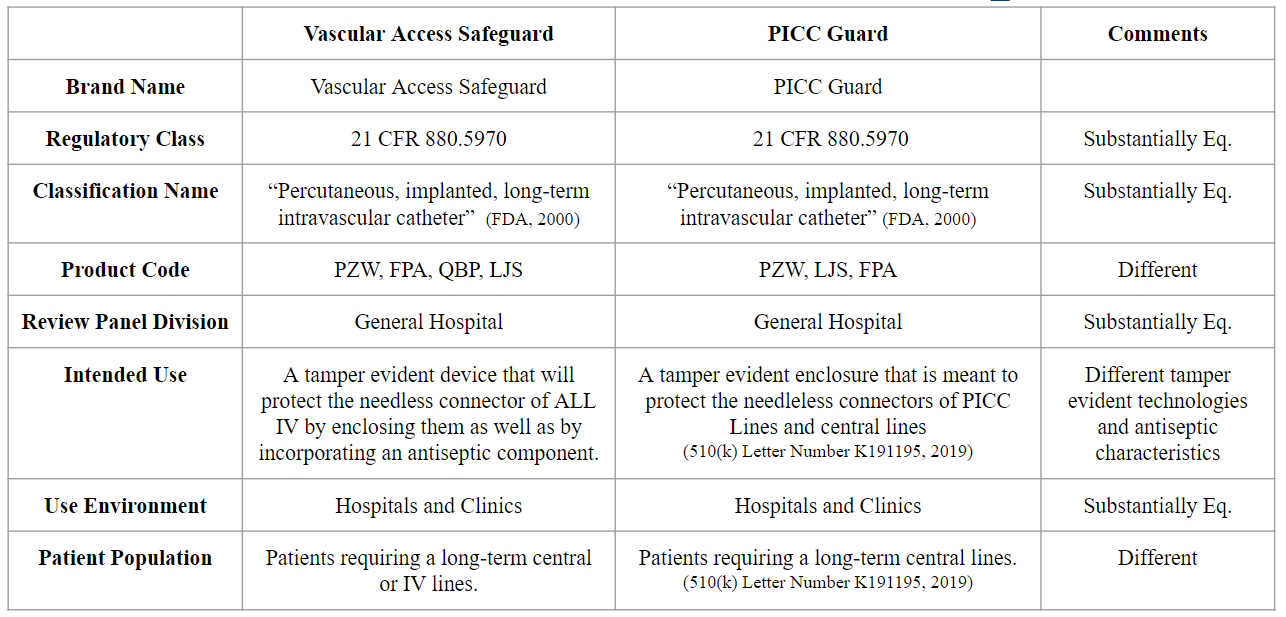
FDA Regulatory Requirements
Acknowledgements
Our team would like to thank the following people for the guidance, feedback, and assistance:
- Professor Scott Seidman
- Professor Amy Lerner
- Joynita Sur, UR Ventures
- Harl Tolbert, UR Ventures
- Jim Alkins
- Marty Gira
- Lynne Brown
- Katherine Sheppard
- URMC nurses who participated in the testing of this device
Team Members




Customer
Kathleen M. Pratt
BS RN, RT(T), Level III, URMC
Supervisor
Michael Giacomelli
PhD, Biomedical Engineering, UR
Project Liaison
Abbi Miller
CMTI, Biomedical Engineering, UR
References
- Ahrq.gov. (2012). Infections Avoided, Excess Costs Averted, and Changes in Mortality Rate. [online] Available at: https://www.ahrq.gov/hai/cusp/clabsi-final-companion/clabsicomp4c.html. [Accessed 27 Oct. 2021]
- Barton, A. (2020). Medical adhesive-related skin injuries associated with vascular access: minimising risk with Appeel Sterile. British Journal of Nursing, 29(8), pp.S20–S27
- “PICC Guard.” PICC Guard, www.piccguard.com/. Accessed 18 Apr. 2022.
- Tremblay, T. (2012). Injection Moulding Part Design for Dummies. [online] Hoboken, NJ: John Wiley & Sons, Inc. Available at: https://www.protolabs.co.uk/media/1011290/im-for-dummies-en.pdf [Accessed 12 Apr. 2022]
- www.twi-global.com. (n.d.). What is Injection Moulding? – Definition, Types and Materials. [online] Available at: https://www.twi-global.com/technical-knowledge/faqs/what-is-injection-moulding.
- Wisconic, Inc (2019). Considerations for Injection Molding. [online] Wisconic. Available at: https://www.wisconic.com/injection-molding/considerations-for-injection-molding/ [Accessed 12 Apr. 2022].
- Justus, M., Justus J., Justus, K. (2020). TAMPER-EVIDENT ENCLOSURE FOR PICC LINE. U.S. Patent No. US 2020/0391001 A1. Washington, DC: U.S. Patent and Trademark Office
- Rucker, H. (2019). TAMPER RESISTANT CLAMP. U.S. Patent No. US 10,173,018 B1. Washington, DC: U.S. Patent and Trademark Office.
- FDA (2000). CFR – Code of Federal Regulations Title 21. [online] www.accessdata.fda.gov. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=880.5970 [Accessed 18 Apr. 2022].
- U.S. Food and Drug Administration, Center for Drug Evaluation and Research, 510(k) Letter number K191195
- U.S. Food and Drug Administration, Center for Drug Evaluation and Research, 510(k) Letter number K190918.
