Meet the Team

Background
Wedge Deformities: Also known as the Wedge Effect. Characterized by the distraction of the proximal femur and lateralization of medullary nails within the femur.
Why is it a Problem?
- Fracture becomes fixed in varus reduction
- Changes biomechanical fixation parameters
- Leads to implant failure or revision surgery
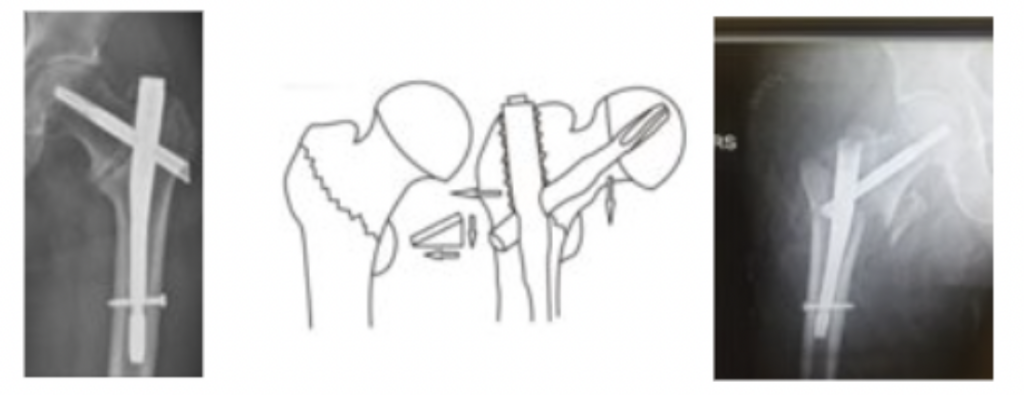
Clinical Scenario
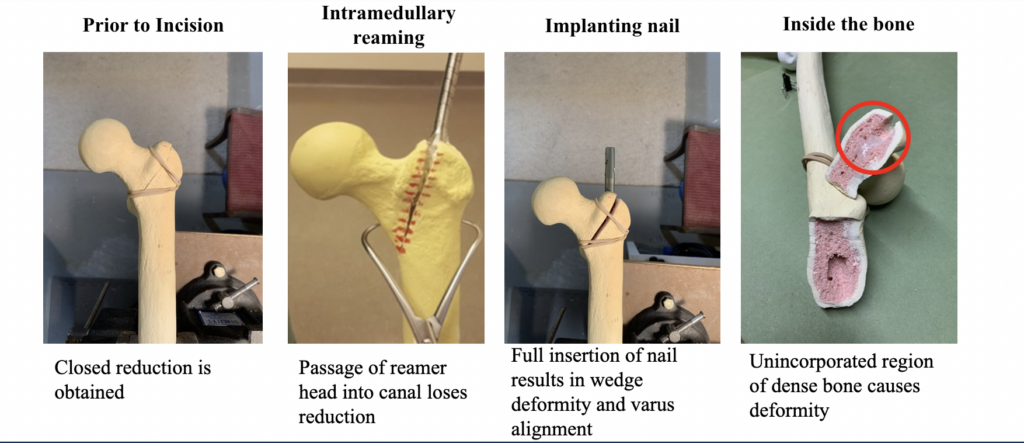
How Does it Happen?
- Region of dense bone in superolateral femoral neck deflects path of reamer
- Only visible upon full insertion of medullary nail, surgeon must repeat reaming step
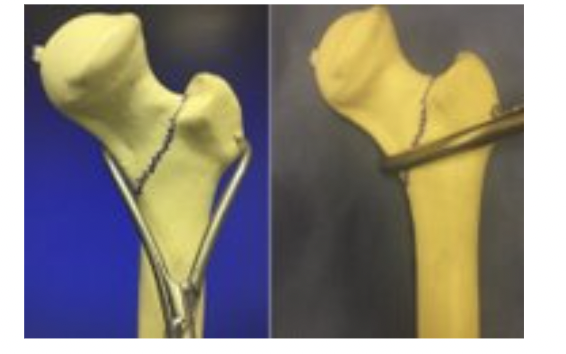
Gaps in Current Solutions:
- Increased physiological burden
- May require an assistant to manually hold instruments
- Solutions are implemented only after the problem occurs
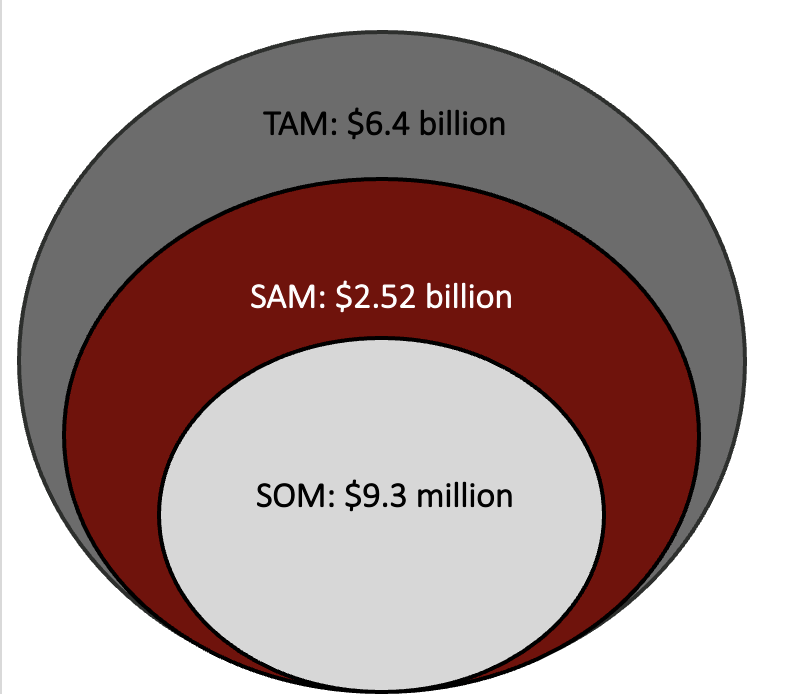
Market Opportunity
- Total addressable market: $6.4 billion global hip fracture treatment
- Serviceable available market: $2.52 billion in spent on costs associated with hip fracture treatment with IMN
- Serviceable obtainable market: $9.3 million (1800 devices and 150,000 disposables)
The Solution
The WedgeWise Clamp: An orthopedic clamp for reducing fragments of the proximal femur during IM reaming
Components:
A: Stainless Steel Head Piece
B: Stainless Steel Nut
C: Polyethylene Body
D: Polyethylene Handle
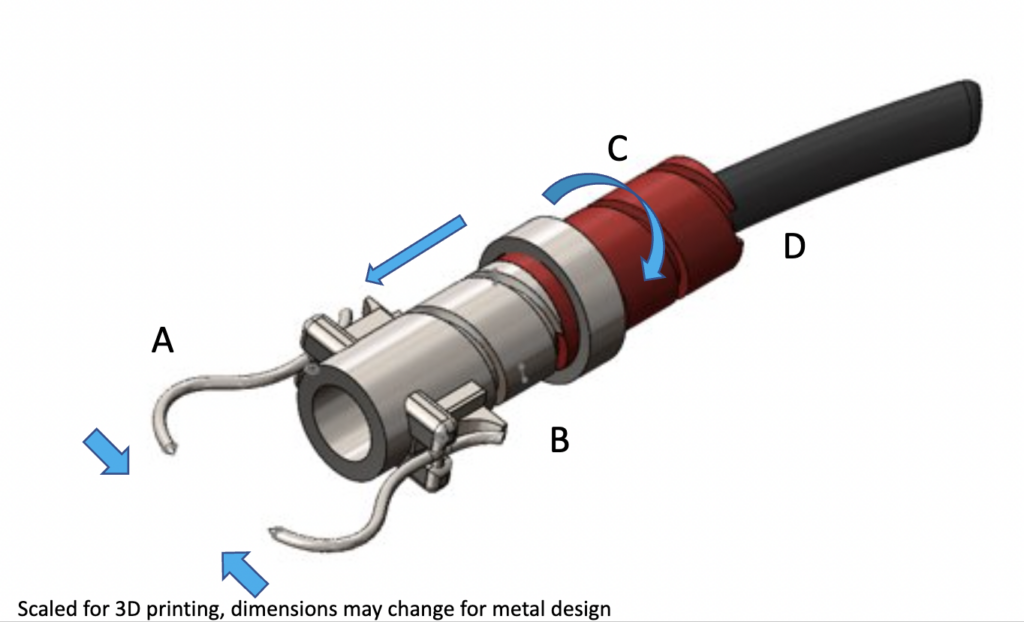
How it Works:
- Tightened into place around tip of trochanter
- Body component extracted
- Handle and body removed from surgical site
- Cannulation allows reaming to take place unobstructed
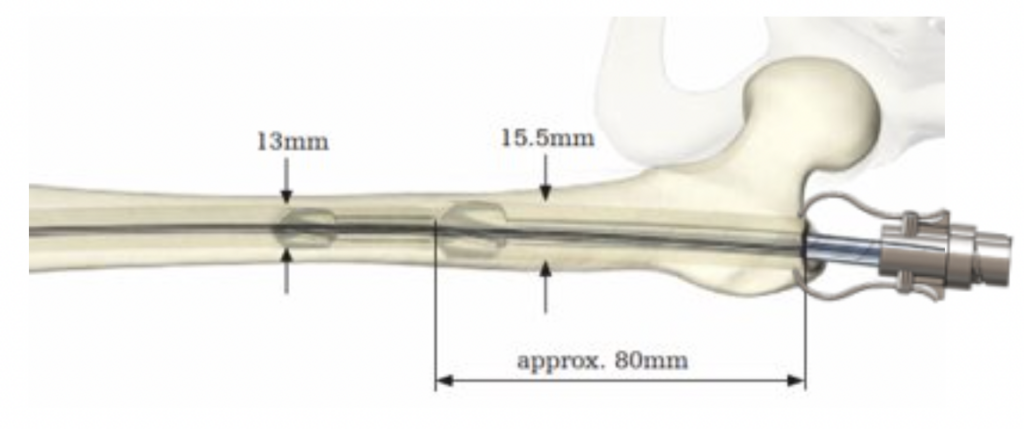
Advantages:
- No additional incisions
- Does not require an assistants help
- Designed to prevent wedge deformities from occurring in the first place
Proof of Concept Testing
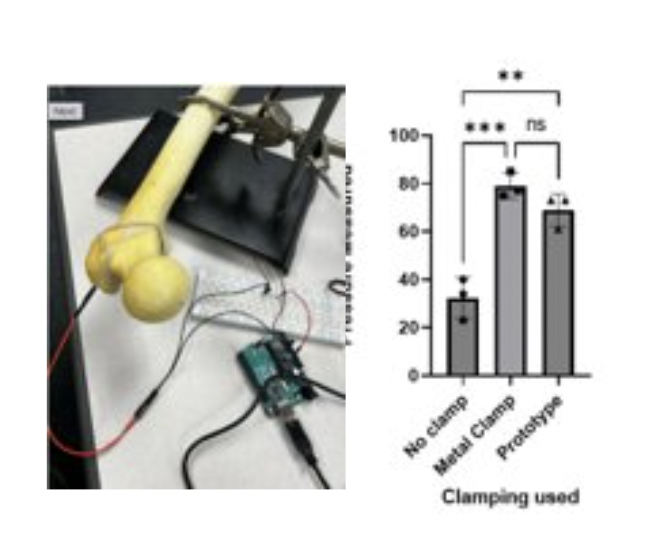
Comparing Clamping Forces: Placing a calibrated pressure sensor in the wedged bone fracture and taking repetitive measurements for the different clamping methods.
Results: Significant difference between clamping and no clamping Non-significant difference between metal clamp and our prototype
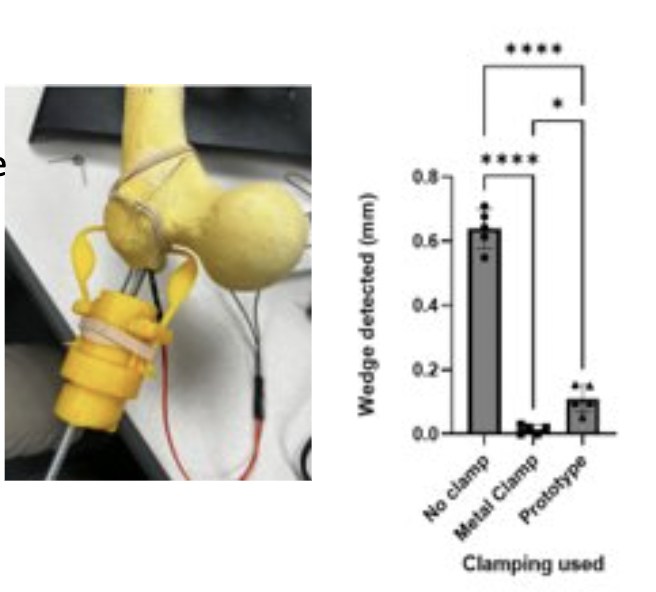
Comparing Wedge Size Detection: Placing a calibrated pressure sensor with known reference values to wedge size and taking repetitive measurements for the different clamping methods
Results: Significant difference between clamping and no clamping for P ≤ 0.0001 . Significant difference between the metal clamp and prototype for P ≤ 0.05
Next Steps
Regulatory Pathway:
- Class II Device – CFR 878.4800, product code -HXD
- 510(k) Pathway
Testing:
- FDA required testing following the Standard Specification and Test Methods for External Skeletal Fixation Devices, cortex clamp ( ASTM F 1541, 6.1.4 )
Future improvements:
- Machined prototyping
- Anatomical validation testing
- Clinical Research
Acknowledgments
- Greg Gdowski (Executive Director of CMTI)
- Marty Gira (Senior Research Engineer
- Amy Lerner (Professor of Biomedical Engineering)
- Dr. Sandeep Soin (Orthopedic Surgeon)
- Dr. David Mitten (Orthopedic Surgeon, Business Coach)
