Part 1: Introduction
Project Motivation
The LiDestri facility processes hundreds of different beverages and spirit products through eight lines. Due to the nature of their quickly changing environment, they produce inconsistent and difficult to characterize wastewater. While the company faces issues with maintaining an appropriate pH (between 5-12), the financial cost relating to the excess of biochemical oxygen demand (BOD) as well as the complexity of their wastewater are the main focuses of this project.
Team Members
 Katerina Connor | kconnor7@u.rochester.edu LinkedIn Profile B.S. ChE, Class of 2021 |
 JunSeok Jeon | jjeon6@u.rochester.edu LinkedIn Profile B.S. ChE, Class of 2021 |
 Connor Pope | cpope5@u.rochester.edu LinkedIn Profile B.S. ChE, Class of 2021 |
 Brian Yegela | byegela@u.rochester.edu LinkedIn Profile B.S. ChE, Class of 2021 |
Project Sponsor

Project Mentors
| UR ChE Mentor: | Prof. Doug Kelley |
| LiDestri Sponsors: | Chris McCormick Fabio Scuruchi Randy Kehl Peter Tadio |
Part 2: Project Background
Background Overview
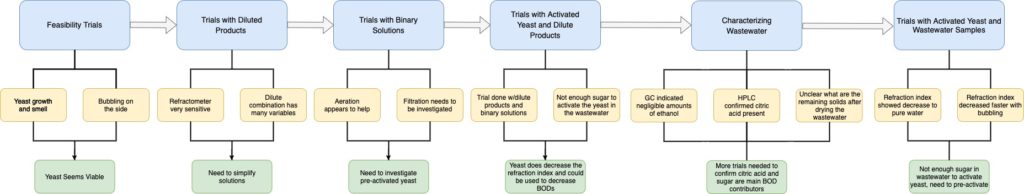
The processing of hundreds of different products through eight manufacturing lines creates inconsistent and arduous wastewater characterization. Treatment, or lack thereof, results in various sewer surcharges based on the excess of materials that make wastewater difficult to dispose of. These materials include biochemical oxygen demand (BOD), total suspended solids (TSS), fats, oils, and grease (FOG), ethanol, and numerous other components. Based on conversations with the main stakeholder of this project, LiDestri, the team decided to characterize various lines and products. The aim of this was to understand the waste streams and identify the most significant contributor to the BOD problem. The team also explored various techniques to reduce the BODs and made a recommendation on a prototype that would aid in mitigating cost.
Project Requirements
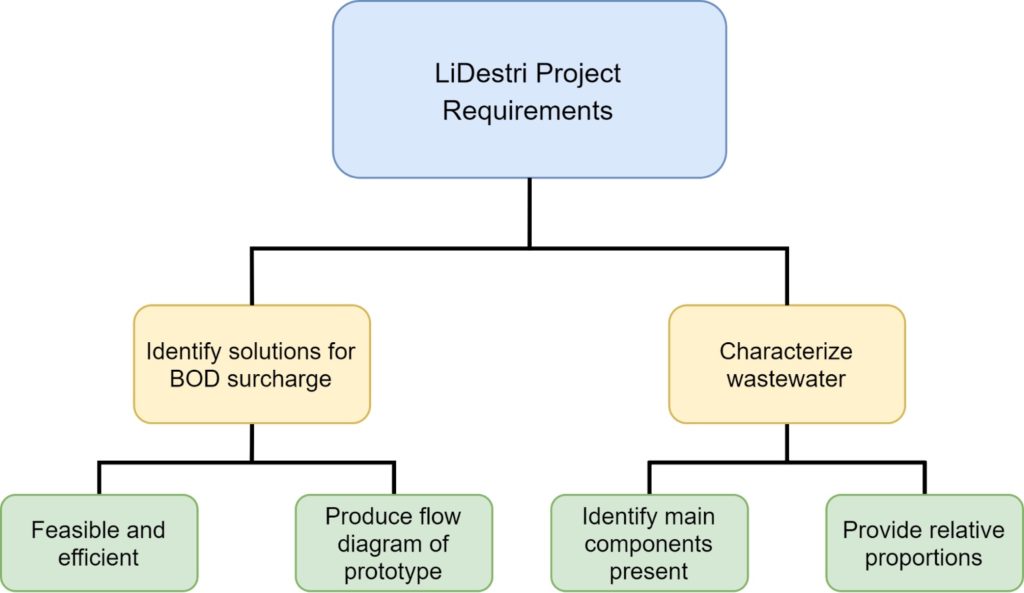
Outline of requirements as described by the sponsor, LiDestri
Part 3: Technical Approach
Yeast Selection and Activation Profile
The yeast chosen to perform trials were selected based upon criteria including fermentation rate, flocculation ability, and temperature range. Four yeast strains were used throughout this project including two wine yeast (EC-1118, K1-V1116) and two beer yeast (Saflager S23, Safale S04). These yeast were then activated using a similar profile to initial beer wort and initial wine, with a specific gravity or concentration of sugar in the solution.

Activated and Unactivated Yeast Trials
For addressing the BOD surcharge concern, the team first investigated yeast as a potential solution. Prior to delving too far into the proposed yeast approach, it was essential to test its feasibility early in the design process. This initial testing is what the team coins “quick and dirty” experiments, which were strictly visual investigations to determine if yeast would react within a simulated wastewater environment. The team then used activated and unactivated yeast for trials using dilute product combinations (designed to try and mimic wastewater), a dilute chocolate cream liqueur, and actual wastewater.

Trials using unactivated yeast
Characterization Approach
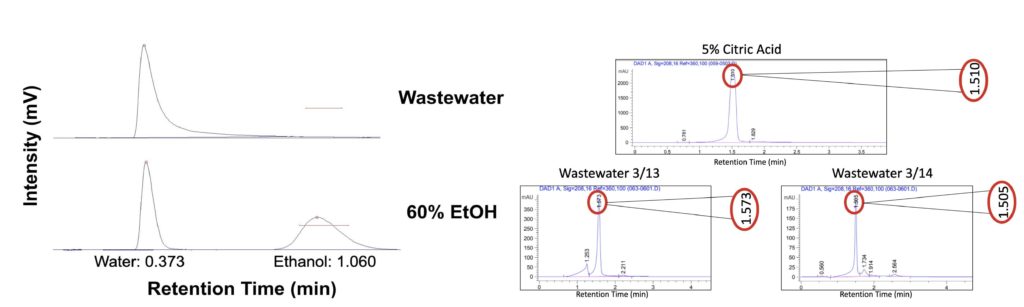
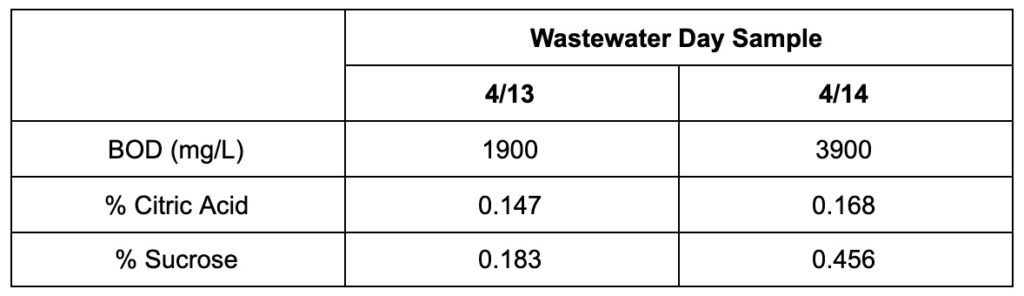
For characterizing the wastewater provided by LiDestri, Team Riddikulus employed various characterization techniques to test for specific components present. After perusing ingredient labels and discussions with the LiDestri team, it was determined that the principal components present in the wastewater were sucrose, ethanol, citric acid, and acetic acid. A word of caution is necessary due to the fact that the wastewater is heavily dependent on which lines are currently in operation, where the team was only able to characterize two days of wastewater.
The first method was coined as the brute force separation method, where the wastewater was filtered to remove any suspended solids. Then, gas chromatography (GC) was used to determine the relative significance of ethanol within the wastewater. High performance liquid chromatography (HPLC) was used to identify the dissolved components within the wastewater, principally acetic acid and citric acid. Sucrose cannot be detected in the HPLC, thus this was not considered for this instrument.

Brute force separation looking
at dissolved solids
Brian prepares the
GC for ethanol detection
JunSeok designs the HPLC program
Finally, the refractometer was used as a quick quantitative measure as this can be used to determine the “purity” of water, where dissolved components will have a refraction index greater than that of water. This characterization approach was used primarily for the activated and unactivated yeast trials. When the wastewater was investigated with yeast, the HPLC was also used to identify the citric acid concentrations after yeast treatment.

Connor (left) and Katerina (right) perform wastewater treatment using yeast. 
Katerina investigates the refraction index of a sample.
Part 4: Results
Yeast Findings & Characterization of Wastewater

Early design development for testing yeast indicating bubbling and foaming
Activated and Unactivated Yeast Trials with LiDestri products combination
- Activated yeast showed a faster decrease in the refraction index when compared to the unactivated yeast counterpart across all trials. Note pure water has a refraction index of 1.333.
- For example, looking specifically at the chocolate cream liqueur solutions, after 20 hrs., the refraction index was lower for the activated yeast compared to the unactivated with values of 1.335 and 1.337, respectively.
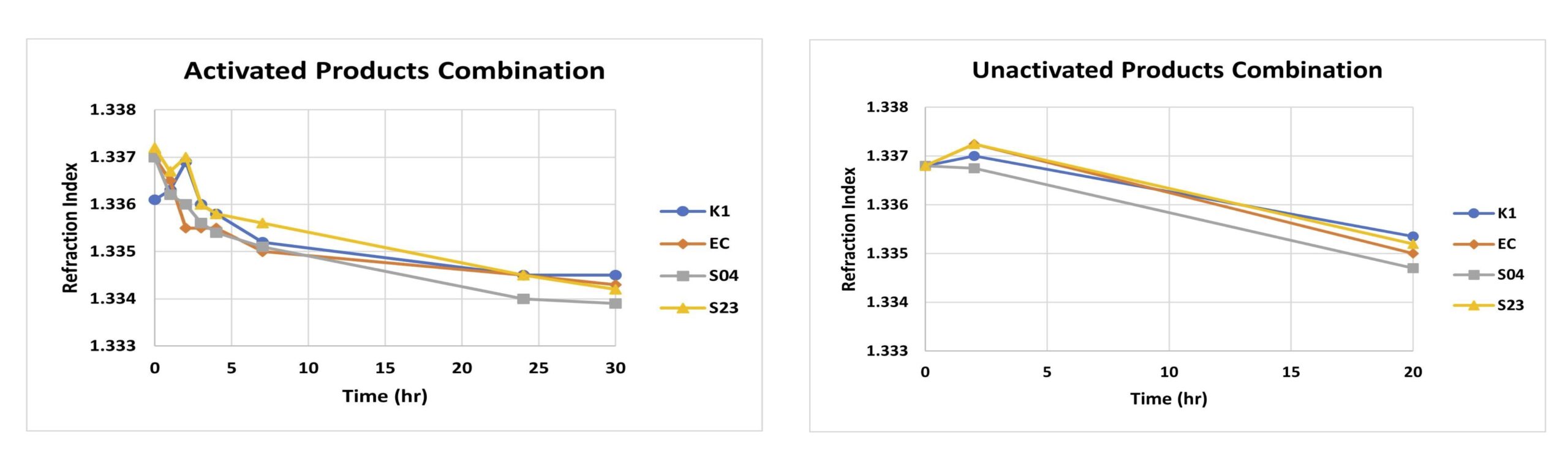
Figures show the comparison of activated vs unactivated yeast using a dilute chocolate cream liqueur sample
Characterizing Wastewater
Data provided from the GC and HPLC during wastewater characterization

Dried wastewater sample indicating crystallization
- Dried wastewater sample indicating crystallization.
- GC results revealed the absence of an ethanol peak, even when the integration factor was set substantially low.
- HPLC results revealed that citric acid was a principal component within the wastewater
- “Brute force separation” method showed about 1% dissolved solids within the wastewater samples
- While this method will not provide quantitative data for what is dissolved, it did show significant crystallization, indicating sucrose was present
- Acetic acid was not detected as its retention time was found to be much later coupled with the fact that LiDestri was not operating its vinegar line during the testing period and acetic acid is the main component in vinegars
- Using BOD values provided by LiDestri, percent sucrose by weight could be calculated
Activated Yeast with Wastewater
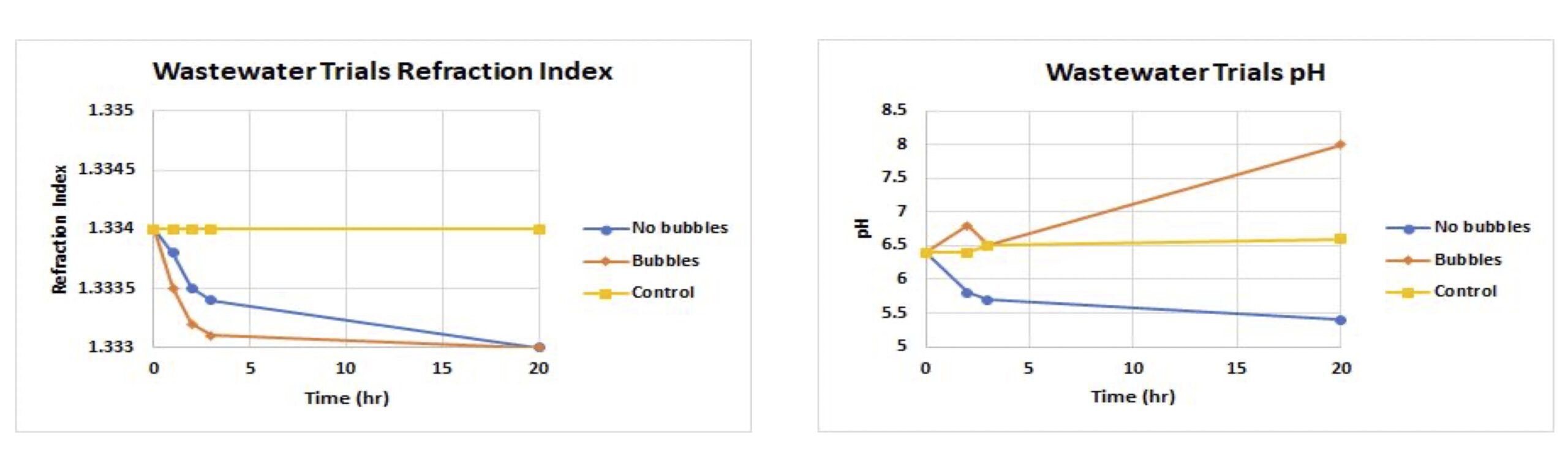
- Bubbling trial was found to have a faster reduction in refraction index due to the agitation generated
- After five hours, the refraction index had decreased significantly, thus this residence time would be used when creating a scaled model
- Lack of aeration saw a decrease in pH over time due to acid formation
- Aeration showed an interesting increase in pH, which is likely caused by a different metabolic pathway where the yeast consumes citric acid, after the sucrose, to produce bicarbonates
- A decrease in citric acid was also observed from HPLC
Characteristics of wastewater when treated with activated yeast using aeration or no aeration
Experiment Overview
General findings and overview of all conclusions
Prototype
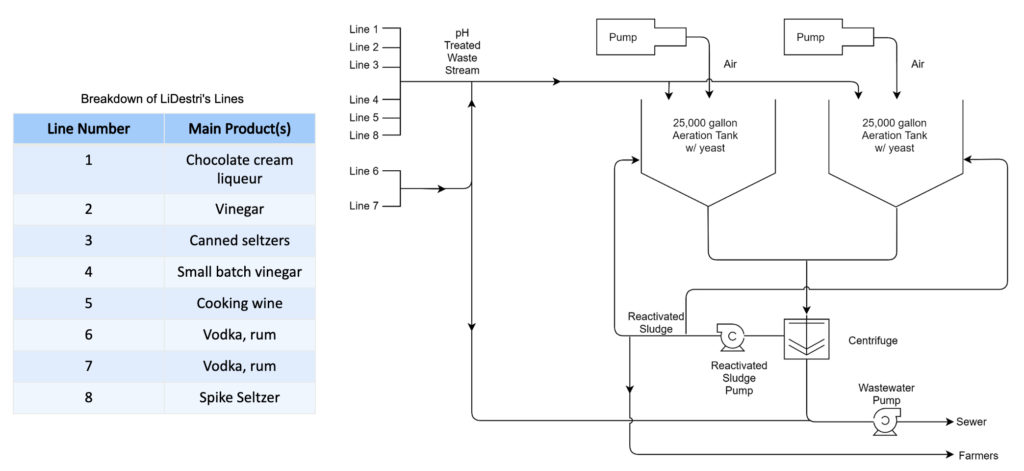
Proposed prototype to be used for wastewater treatment.
Yeast Scale-up
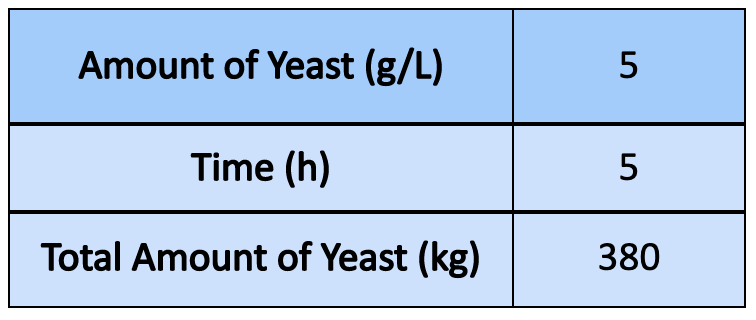
- With regards to scaling up the wastewater treatment system, we estimated a suitable amount required for a larger treatment process
- From the lab trials, it was evident that more time would allow for less yeast to be used. Based off a 5 hour running time, the process allowed for the refractive index of the wastewater provided by LiDestri to approach that of pure water, and this was projected to be a suitable running time
- Once scaled up to 5 g/L, it was found approximately 470 kg of yeast could be used if the aeration tank is completely filled (25,000 gallons, hypothetically). However, this amount is larger than what the actual amount would be, ie. to allow for head space in the tanks to accommodate other factors in yeast growth, bubbling, and safety measures to be considered
- To scale the amount of yeast to industrial size, 80% volume of the tank (20,000 gallons) was used.
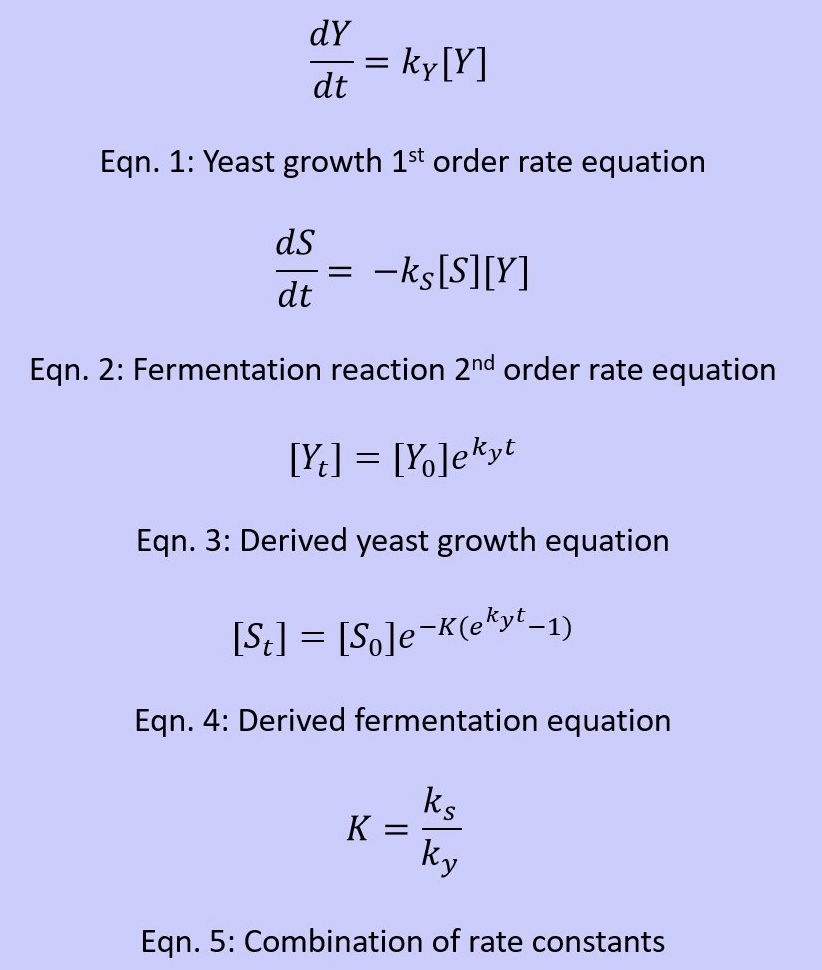
Reaction Kinetics
- We based the growth of yeast on a first order reaction and further the consumption of sucrose as a fermentation process in 2nd order. It is important to note that in this situation, the exponential growth for yeast as modeled is based off of a short period of time
- Variables: [Y] =Yeast Concentration, [S] = Sucrose Concentration, ky = Yeast Rate Constant, ks = Sucrose Rate Constant, t = time
- There is a lag phase, an exponential or a log phase, and eventually there is a stationary phase or in this perspective it is a death phase, and this is the point at which the yeast no longer consumes or has nothing left to consume
- These equations are based off an exponential phase. Using the data acquired from the yeast activation process with sucrose we were able to evaluate the rate constants for this exponential phase
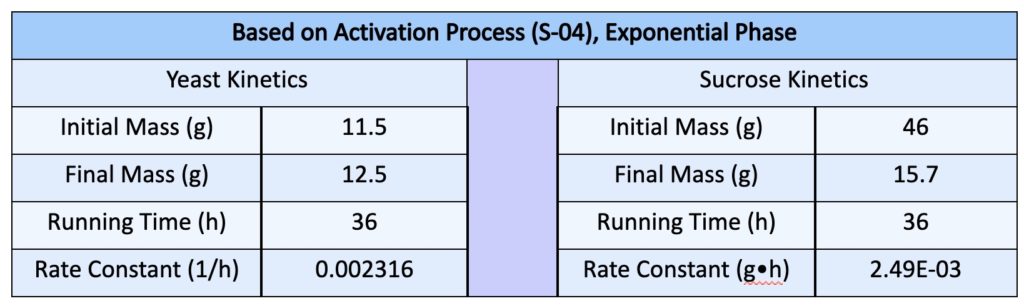
- We used the refractive index to evaluate the final sucrose concentration which was then used to evaluate masses
- Since we were already set to do an activation of the yeast for use in the trials with wastewater, we thought that it would be helpful to record the quantities to model our system and evaluate the constants
- The yeast grew in mass by a single gram over the span of 36 hours and the rate constant was evaluated to being ~ 0.002 h-1 which proposed the relatively slow growth of yeast over a span of 36 hours
- Note that the rate constant evaluated for yeast is based on the exponential relationship that was evaluated previously. This is actually limited by the availability of nutrients, in this case, sucrose. Another method would be to incorporate a parameter for sucrose that could be used to solve for the rate constants numerically.
- Note that the relationship of consumption is not linear, and is actually exponential. Consequently, the majority of the reaction can be accomplished in the initial zone of the run times
Part 5: Conclusions & Future Work
Conclusion
The team achieved significant findings that can aid LiDestri in its wastewater management. The team was able to identify the significant components in the waste stream: sucrose and citric acid. While the facility does process many high-proof liquors and malt beverages, ethanol was not shown to be a significant component in the wastewater. Both sucrose and citric acid play a role in the BOD surcharge, consequently, coupled with the team’s novel approach to addressing this issue, the team was effectively able to reduce the refraction index of sample wastewater, which is directly correlated to the BOD content. This novel approach included the addition of activated yeast in an aerobic environment. The team was able to produce an industrial scaled model using kinetic calculations. Finally, it was determined that any brewer’s yeast (beer or wine) would suffice for reducing the BOD value.
Future Work
The team was able to accomplish the intended goals set out in the early stages of the project as well as adapt to the customer’s changing needs. The figure below outlines the proposed future work for this project. One consideration is to vary the amount of yeast added, which can be used to determine the optimal amount of yeast for reducing the BODs in the industrial sized prototype. For better characterization of the waste, the team proposes testing and characterizing individual product waste streams to determine if some streams can bypass the aeration tanks. For a sustainable and cost effective solution, the team recommends connecting with local breweries for sourcing the initial yeast as well as connecting with local farmers for yeast disposal (as a livestock foodsource).
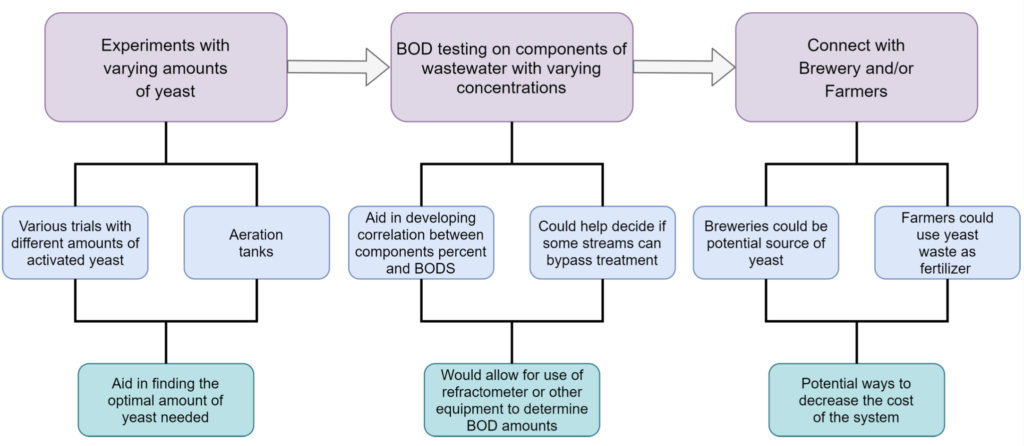
General outline for continuation and expansion of the current findings.
Acknowledgements
We would like to acknowledge the support of the entire ChE 255 team highlighting the help from our mentor Prof. Doug Kelley and our TA John Lovito. We would also like to thank our project sponsors at LiDestri including Peter Tadio, and Randy Kehl, and especially Fabio Scuruchi and Chris McCormick. These figures have been instrumental to the success of this project.

The team looks forward to properly disposing the samples provided
References
Citric Acid Safety Data Sheet. Whitaker Oil, 21 May 2018, www.whitakeroil.com/wp-content/uploads/2018/06/Citric-Acid.pdf.
Davidson D, Eggleton P, Foggie P. The diffusion of atmospheric gases through fats and oils. Q J Exp Physiol Cogn Med Sci. 1952;37(2):91-105. doi: 10.1113/expphysiol.1952.sp000986. PMID: 14949320.
Heukelekian, H., and M. C. Rand. “Biochemical Oxygen Demand of Pure Organic Compounds: A Report of the Research Committee, FSIWA.” Sewage and Industrial Wastes, vol. 27, no. 9, 1955, pp. 1040–1053. JSTOR, www.jstor.org/stable/25032868.
Office of Industrial Waste Control. (2019, October 1). Initial Industrial Sewer Use Permit [PDF]. Rochester: Monroe County Department of Environmental Services.
Refractive Index. (n.d.). Retrieved March 22, 2021, from http://faculty.weber.edu/ewalker/Chem2990/Chem%202990%20Refractive%20Index%20Readings.pdf
Refractive Index of Ethanol–Water Mixtures and Density and Refractive Index of Ethanol–Water–Ethyl Ether Mixtures. (1946) Troy A. Scott The Journal of Physical Chemistry 50 (5), 406-412 DOI: 10.1021/j150449a003
Sparhawk A. Defining Beer Gravity. CraftBeer.com. https://www.craftbeer.com/craft-beer-muses/defining-gravity. Published August 25, 2016.
Toikkanen, Outi & Lähteenmäki, Maija & Moisio, Timo & Forssell, Pirkko & Partanen, Riitta & Murtomäki, Lasse. (2014). Study of Oxygen Transfer across Milk Proteins at an Air–Water Interface with Scanning Electrochemical Microscopy. Journal of agricultural and food chemistry. 62. 10.1021/jf5008715.
Using a Hydrometer. Love Brewing – Home Brew Guides & Videos. https://www.lovebrewing.co.uk/guides/using-a-hydrometer/. Published December 24, 2020.
Valtech Diagnostics Inc: Ethanol Alcohol, 95% v/v Safety Data Sheet, Issued 2014 http://www.labchem.com/tools/msds/msds/VT230.pdf
Wang, Yan & Qiu, Liping & Hu, Mengfei. (2018). Application of yeast in wastewater treatment. E3S Web of Conferences. 53. 04025. 10.1051/e3sconf/20185304025. Yakobson C. The Oxford Companion to Beer Definition of flocculation. Craft Beer & Brewing. https://beerandbrewing.com/dictionary/9yHbaDo6RA/#:~:text=Flocculation%20is%20the%20tendency%20of,end%20of%20the%20fermentation%20process.