Testing & Scale-up
To further understand and gauge the device, Viriotech tested the cassette in three categories: evaporation, sealability, and usability.
Evaporation
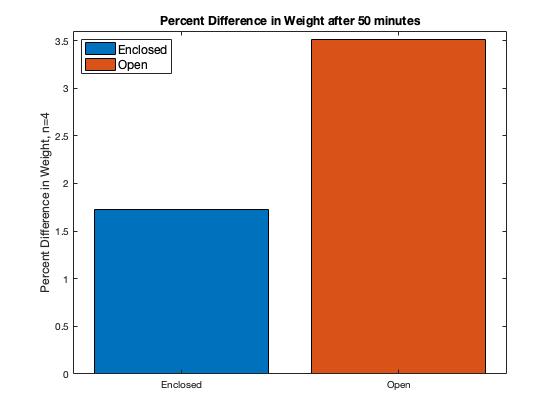
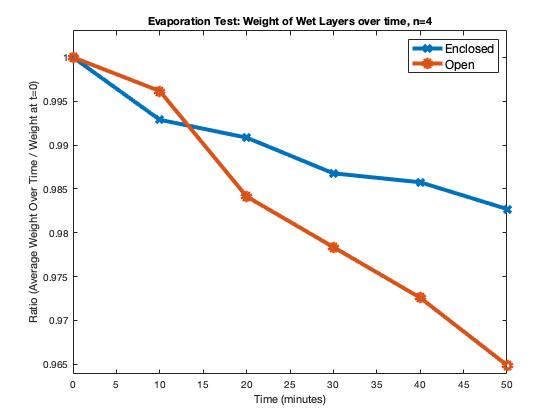
The HIV assay runs on capillary action, so enough blood must run through the layers during the test. Evaporation tests were done to ensure the cassette protected the fluid from the environment. The figure below shows (1) the percent difference in the weight of the layers after 50 minutes and (2) the fluctuations of the weight over time. Four-layer assemblies were used for the tests in two environments: enclosed within the cassette and open/exposed to the environment. Results show that the evaporation rate is lower in the enclosed layers. The data demonstrate that our cassette prevents the evaporation of blood samples, but not perfectly. Nonetheless, we estimate that a less than 2% weight change does not harm the final viral result.
Sealability
The sealability of the device is currently being tested in the following conditions:
- Bumped cassette (moving left and right, up and down, forward and back, pitch, roll, and yaw)
- Dropped cassette (0-6 feet with intervals of 0.5 feet) onto a floor of different textures (granite, wood, mud).
- Slide rotator test: fill cassette with 750 uL of water, close lids, and rotate the cassette at four different speeds (20 rpm, 40 rpm, 60 rpm, & 80 rpm) for 30 seconds, and then check for any leaks.
Results from the slide rotator test:
20 RPM
Results: No leaks
40 RPM
Results: No leaks
60 RPM
Results: No leaks
80 RPM
Results: No leaks
Usability
The usability of the device and the L-TOE were determined by peers who are either experienced with clinical work or are not. They were each handed the cassette and the L-TOE without any instructions, and then asked to assemble the cassette with the L-TOE. Here are the results before and after using the protocol.
| Feedback | Clinical Work Experience | No Clinical Work Experience |
| Usability | 9/10 | 7.5/10 |
| Number of mistakes made before provided protocol in previous prototype | 1: fitting in L-TOE | 2: add blood into inlet incorrectly & not properly fitting in L-TOE |
| Number of mistakes made before provided protocol in most recent, presented prototype | 0 | 0 |
| Time taken to understand how to fit the cassette into the L-TOE without protocol/steps | 2-3 minutes | 5-8 minutes |
Other Observations:
- Peers memorized the whole protocol immediately with no mistakes made afterwards
This feedback, mainly on the L-TOE, led us to implement the most recent changes to the device, as featured on the Cassette Design page.
Additionally, the team met with experts in the field to ensure the device has an intuitive workflow and will in fact be used in the setting. These experts include doctors focused on HIV treatment and testing and others working directly in low-resource communities in Eastern Africa. They have encouraged us to create a pictographic pamphlet to prevent language barriers that we will be including in our final report and presentation.
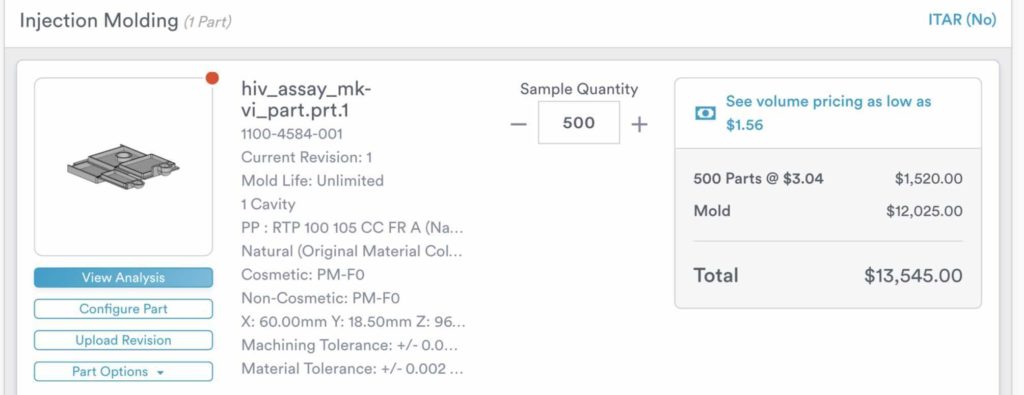
Injection Molding
The cassette will be mass-produced via injection molding which is a mold to shape the plastic, polypropylene, into the cassette design. To get an estimation of the cost of injection molding, the team reached out to ProtoLabs with our CAD drawings of the prototype. The following figure shows the quote from ProtoLabs. The estimated cost of each device is $3.04, while a mold with an unlimited mold life costs $12,025. Overall, it would cost $13,545 to injection mold 500 units. The mold, the most costly component, has an unlimited life and thus would only be an initia, required investment by the manufacturers.