Technical Approach
The technical approach can be broken into two parts:
- Fluid screening for side reactions with high-performance liquid chromatography (HPLC) and UV-Vis
- Use of excess sodium sulfite to calculate oxygen transfer rates (OTR)
Fluid Screening with HPLC/UV-Vis
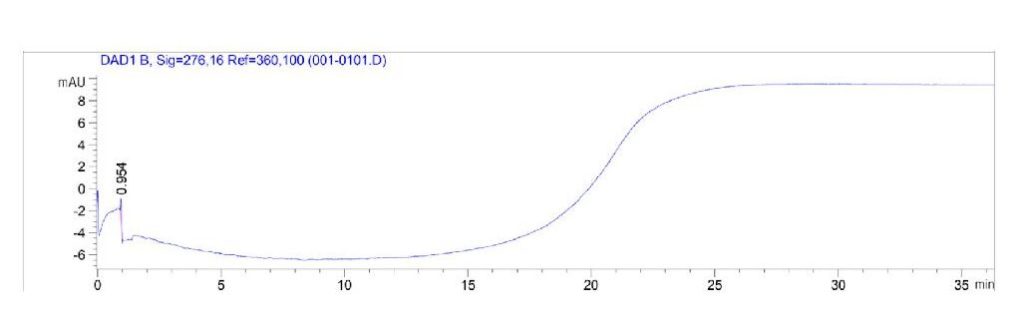
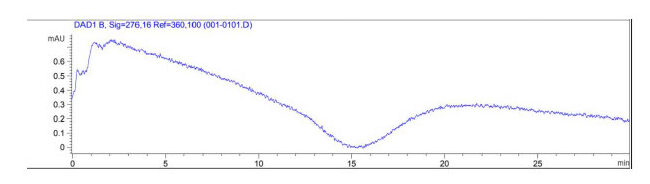
UV-Vis spectroscopy using a HPLC was implemented to determine whether side reactions may occur. This was performed for PEG 3350 and sodium sulfite, as well as ethylene glycol (EG) and sodium sulfite in order to determine if a noticeable shift in absorption occurred. A change in absorption indicates that a reaction occurred, changing the absorbance of the products. An indication of side reaction(s) would present a problem as this would interfere with oxygen concentration data collection.
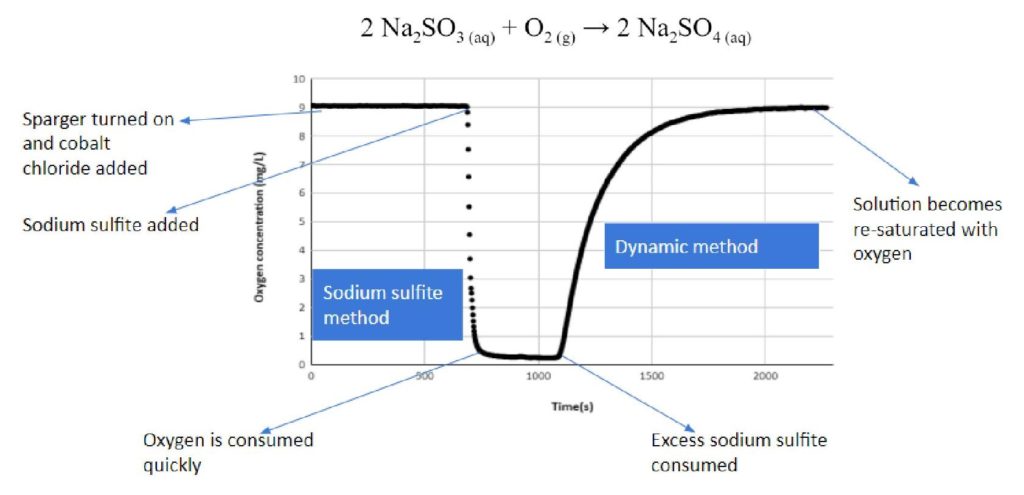
The Steady-State Excess Sodium Sulfite Method
The sodium sulfite method utilizes stoichiometry to measure oxygen concentration drops within the tank. This method was used in conjunction with the dynamic method, which regresses an exponential curve to determine OTR. Interactions with the fluids in the system make it difficult to measure exactly, as the chemical mechanism proceeds by free radical propagation.