News & Events
What do Chemical Engineers do? Just look around you!
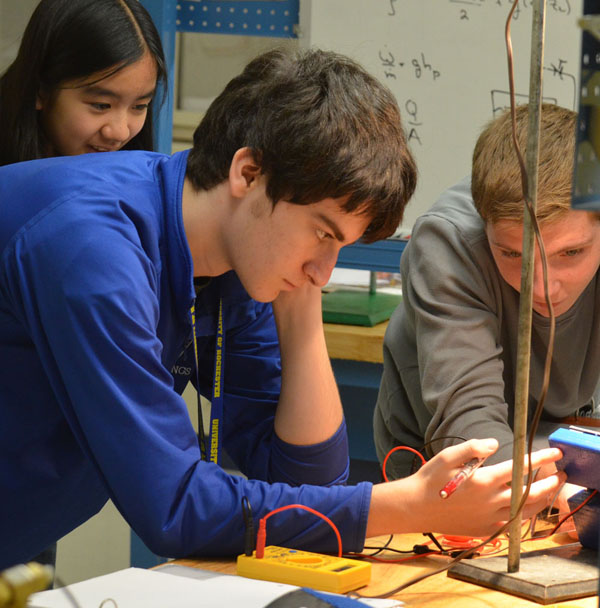 (Aaron G. of Waco, Tex., foreground, and Jonathan T. of Dayton, Ohio, observe how the voltage generated by a solar panel changes depending on its angle to its light source, while Samantha H. of Pittsford, N.Y., looks on. The high school students were among 15 who participated in a Rochester Scholars pre-college course on chemical engineering, taught by Asst. Prof. Wyatt Tenhaeff and Senior Technical Associate Rachel Monfredo, with help from Branden Cole, a ChemE undergraduate. Click here to see more photos of the course.)
(Aaron G. of Waco, Tex., foreground, and Jonathan T. of Dayton, Ohio, observe how the voltage generated by a solar panel changes depending on its angle to its light source, while Samantha H. of Pittsford, N.Y., looks on. The high school students were among 15 who participated in a Rochester Scholars pre-college course on chemical engineering, taught by Asst. Prof. Wyatt Tenhaeff and Senior Technical Associate Rachel Monfredo, with help from Branden Cole, a ChemE undergraduate. Click here to see more photos of the course.)
Chemical engineers modify the polymers that give us nylon for our jackets, vinyl siding for our homes, the latex paint on our walls, the rubber soles on our shoes, and the shampoo, toothpaste and cosmetics that keep us clean and presentable.
Chemical engineers apply fluid mechanics to design pipeline systems that convey chemicals over distances ranging from tens to thousands of meters. They might also design mixing vessels that homogenously disperse these chemicals efficiently. And who makes sure our ketchup is not too thick to squeeze out of a bottle but not so thin it separates before we can use it? Chemical engineers, who study the fluid properties of the ketchup and potentially alter the recipe!
They are employed in industries as varied as petrochemical and microelectronics, pulp & paper and pharmaceuticals.
They are at the cutting edge of technology – and consumer taste. They work on safer, more efficient batteries to boost the mileage of our electric cars, for example, and new ways to combine the ingredients of our favorite breakfast cereals.
“The chemical engineering degree is very diverse,” Assistant Professor Wyatt Tenhaeff told 15 high school students recently during a weeklong Rochester Scholars pre-college introduction to the field. “It sets you up for a lot of opportunities.”
How chemical engineering differs from chemistry
How is a chemical engineer’s job different from that of a chemist?
A chemist is more likely to do pure research, developing new compounds and materials in a laboratory; a chemical engineer is more likely to develop ways to process and manufacture those materials in a chemical plant.
This involves huge differences in scale. A chemist may react a few grams, or even micrograms of different compounds using a flask, condenser and syringe at a laboratory bench. A chemical engineer may be responsible for mixing literally tons of chemicals in batch reactors that may be 20 feet wide and 50 feet tall, or larger. This involves more than just mixing the right proportions of different compounds. It involves myriad engineering skills to ensure the process is both efficient and safe.
Consider styrene. This organic compound, a derivative of benzene, is the starting material, or “feedstock,” for many of our plastic products. There is a huge market for it – 55 billion pounds a year. The two compounds that are combined to produce styrene cost about 72 cents a pound; styrene sells for only 77 cents. And yet, given the demand, that still translates into a potential profit of $2.75 billion a year, Tenhaeff noted. And it’s up to the chemical engineers to make sure that the companies producing styrene maximize every cent of that profit.
How?
By applying thermodynamics to know exactly how much energy will be required to heat the reactants. By using fluid dynamics to determine how large the pipes should be and what they should be made of. By understanding reaction kinetics to determine the optimum size of the reactor tank. And by applying process dynamics to install sufficient safety controls to prevent a catastrophic breakdown.
Chemical engineers are well rewarded
This partly explains why chemical engineers, on average, are paid more than chemists. The average starting salary for chemical engineers is
$67,500, versus $44,700 for chemists. “Chemical engineers make a lot of the decisions and design choices that influence how profitable a chemical process will be,” Tenhaeff explained. “They are much closer to the processes that make money.”
During their weeklong introduction to chemical engineering in July, the students began each morning with a 30-60 minute lecture, introducing them to various general topics and some of the mathematical laws and equations that chemical engineers work with.
“Calculus is integral to chemical engineering,” Tenhaeff noted.
Lab work gives students hands-on experience
The lectures were followed by hands-on experiments in the Department of Chemical Engineering’s recently refurbished undergraduate teaching lab. The laboratory exercises, developed and coordinated by Senior Technical Associate Rachel Monfredo, included synthesizing biodiesel fuel from vegetable oil, and calculating flow rates through different sizes of tubing. Students mixed crystal violet solution with sodium hydroxide in a batch reaction, then used computer-interfaced colorimeters to study reaction kinetics using the relationship between reactant concentration and optical density. They created polyurethane foam and nylon. They evaluated the generation of voltage and current from a solar panel under different exposures to light.
“I really liked the lab work and hands-on activity,” said Riley W. of Medina, Ohio. “We got to make so many things in a short time. I really liked the biofuel synthesis . . . and the solar cells were really cool. It helped me understand the difference between general chemistry and chemical engineering.”
Garret H., of Gananda High School, concurred. “It opened me up to a field that I wasn’t too familiar with, with things I hadn’t seen before, things I didn’t know.” He’s definitely considering a major in chemical engineering – and pursuing it at the University of Rochester.
“UR is up there at the top of my list.”
In the photo below, Taylor D. (left) of Canandaigua, N.Y., and Riley W. of Medina, Ohio, collaborate on an experiment mixing crystal violet solution with sodium hydroxide in a batch reaction, then using computer-interfaced colorimeters to study reaction kinetics using the relationship between reactant concentration and optical density.

